At Pressures Greater Than 1 Atm Water Will Boil At
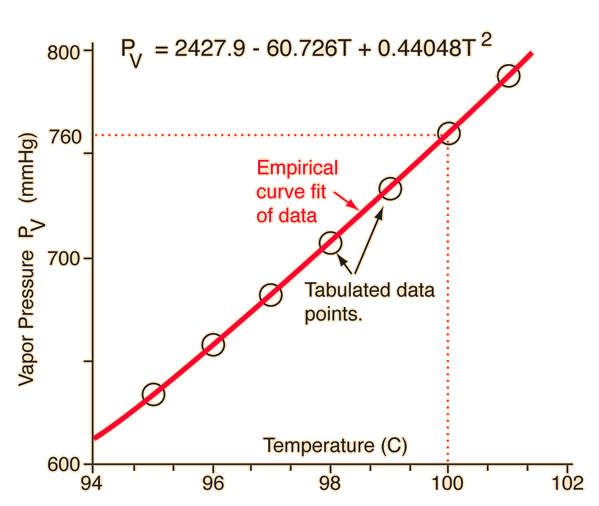
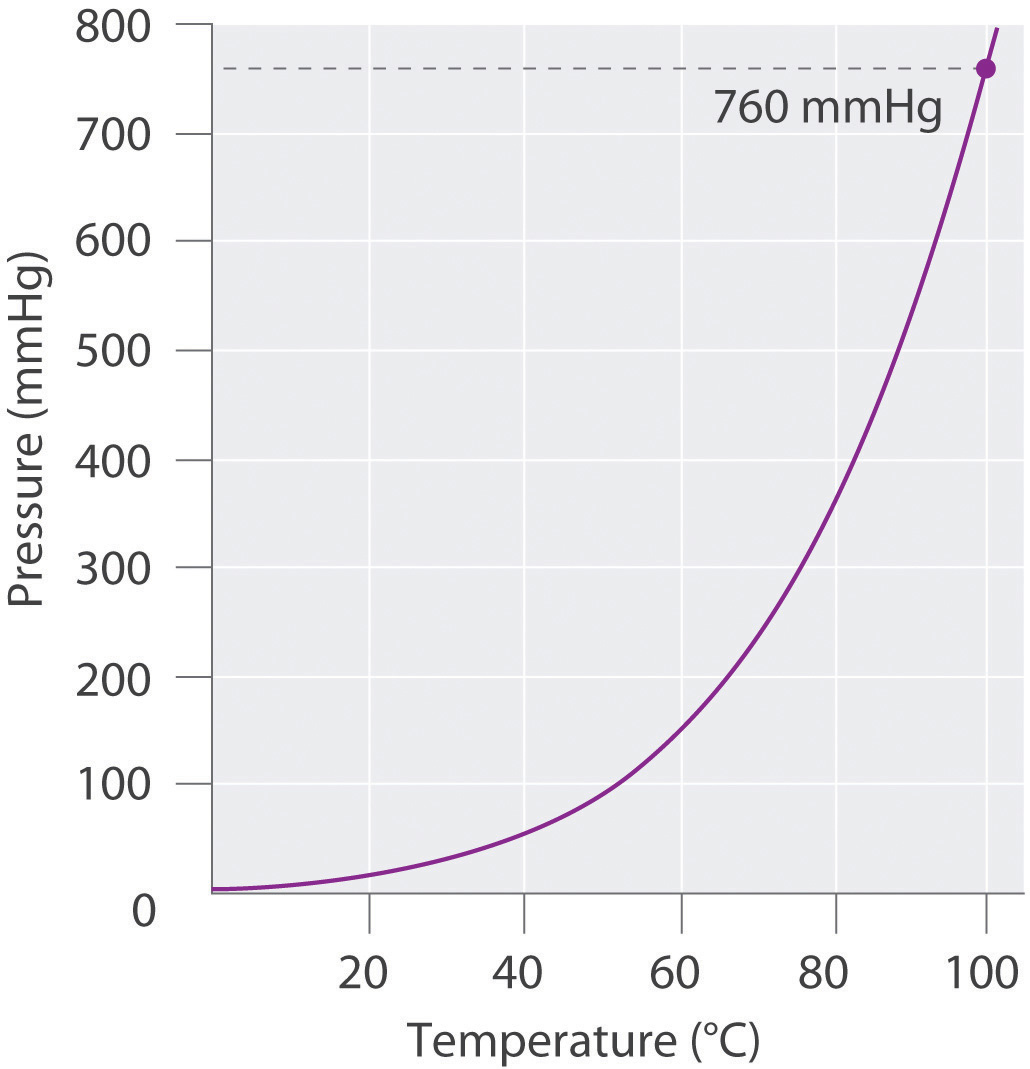
This means that the vapor pressure of water is 1 atm or 760 mmHg at 100 оC.
At pressures greater than 1 atm water will boil at. The equilibrium vapor pressure of a liquid increases with increasing temperature because. A) a temperature less than 100ºC. A temperature higher than 100 C.
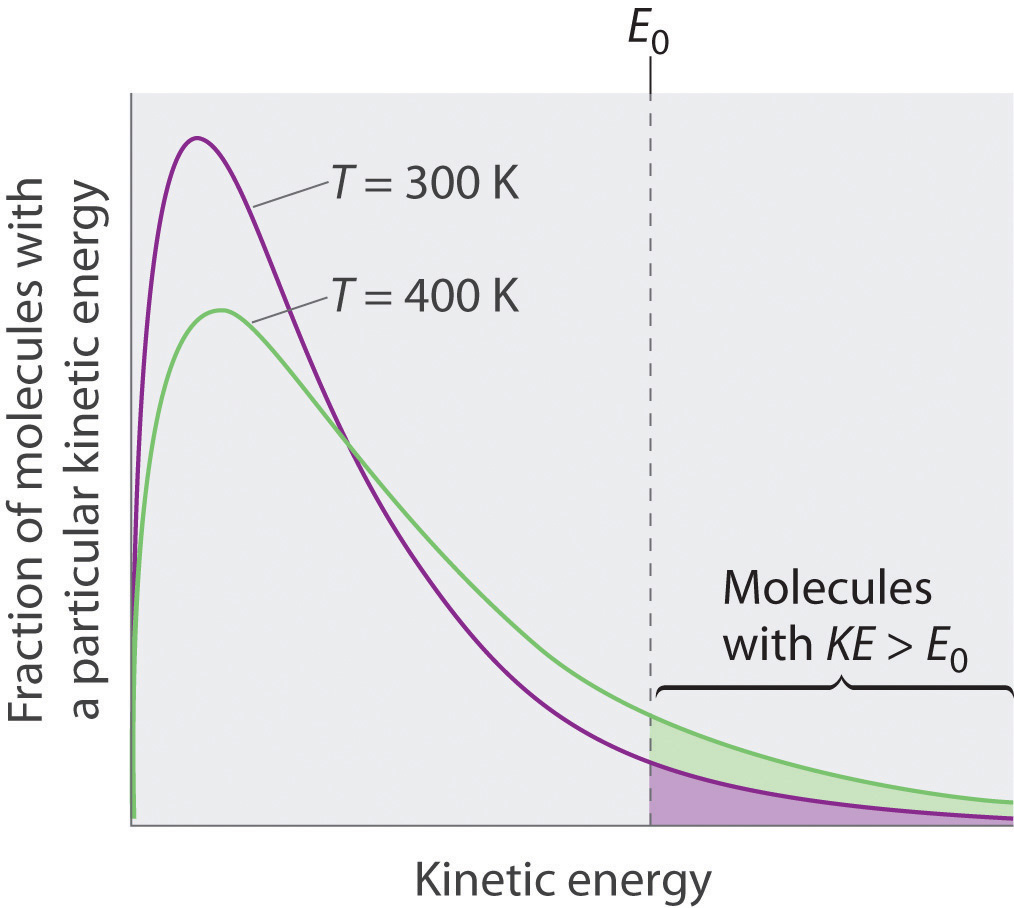
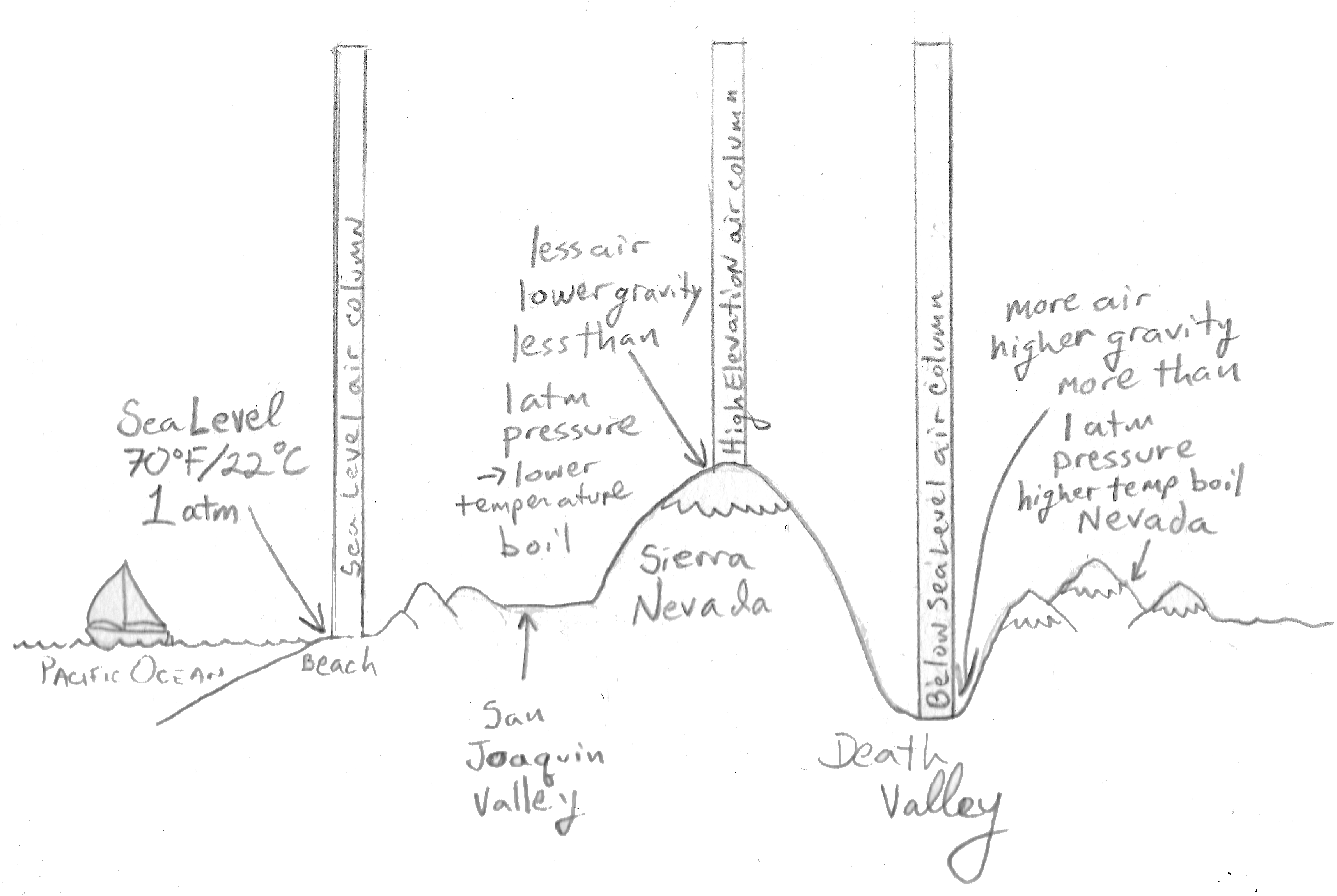
It is well known to mountaineers that it is almost impossible to make a satisfactory. Hence the molecules must have greater kinetic energy to escape from the surface. Hence the molecules must have greater kinetic energy to escape from the surface.
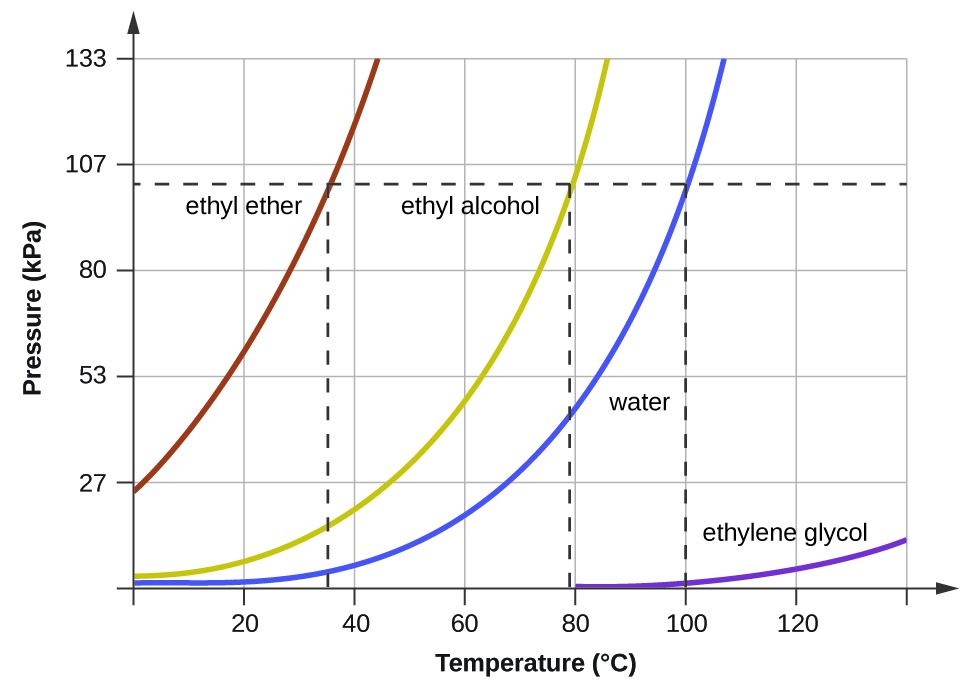
This underlies the principle of vacuum distillation, where an otherwise involatile liquid may be distilled at REDUCED pressure, and the material may be purified somewhat. Conversely, at pressures less than 1 atm, water boils below 100°C. It allows water to boil at 100 °C at pressures greater than 1 atm.
Conversely, at pressures less than 1 atm, water boils below 100°C. To understand it more clearly, recall that the boiling point of water is 100 оC at 1 atm. At a pressure greater than 1 atm, water boils at a temperature greater than 100°C because the increased pressure forces vapor molecules above the surface to condense.
At pressures greater than 1 atm, water will boil at. It allows water to boil at temperatures above 100 °C. It provides very high temperatures and very low pressures.
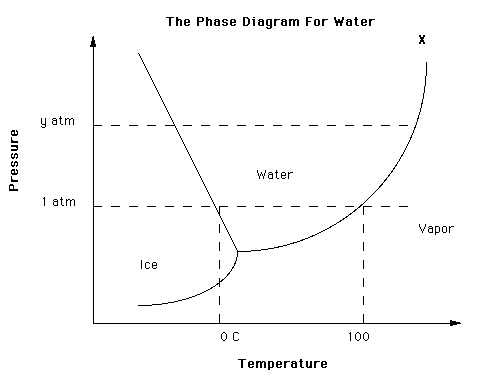
C) a temperature greater than 100ºC In a mountain location where the atmospheric pressure is less than 1.00 atm, water will boil at ___. The normal boiling point is specified when the ambient pressure is 1 ⋅ atm. By definition, the boiling point of a liquid is the conditions of temperature and pressure when the vapour pressure of the liquid is equal to the ambient pressure and bubbles of vapour form directly in the liquid.
The average energy of the particles in the liquid increases. At a pressure greater than 1 atm, water boils at a temperature greater than 100°C because the increased pressure forces vapor molecules above the surface to condense. At places like these, water boils more quickly than in higher pressure areas close to sea level, but a lower temperature.
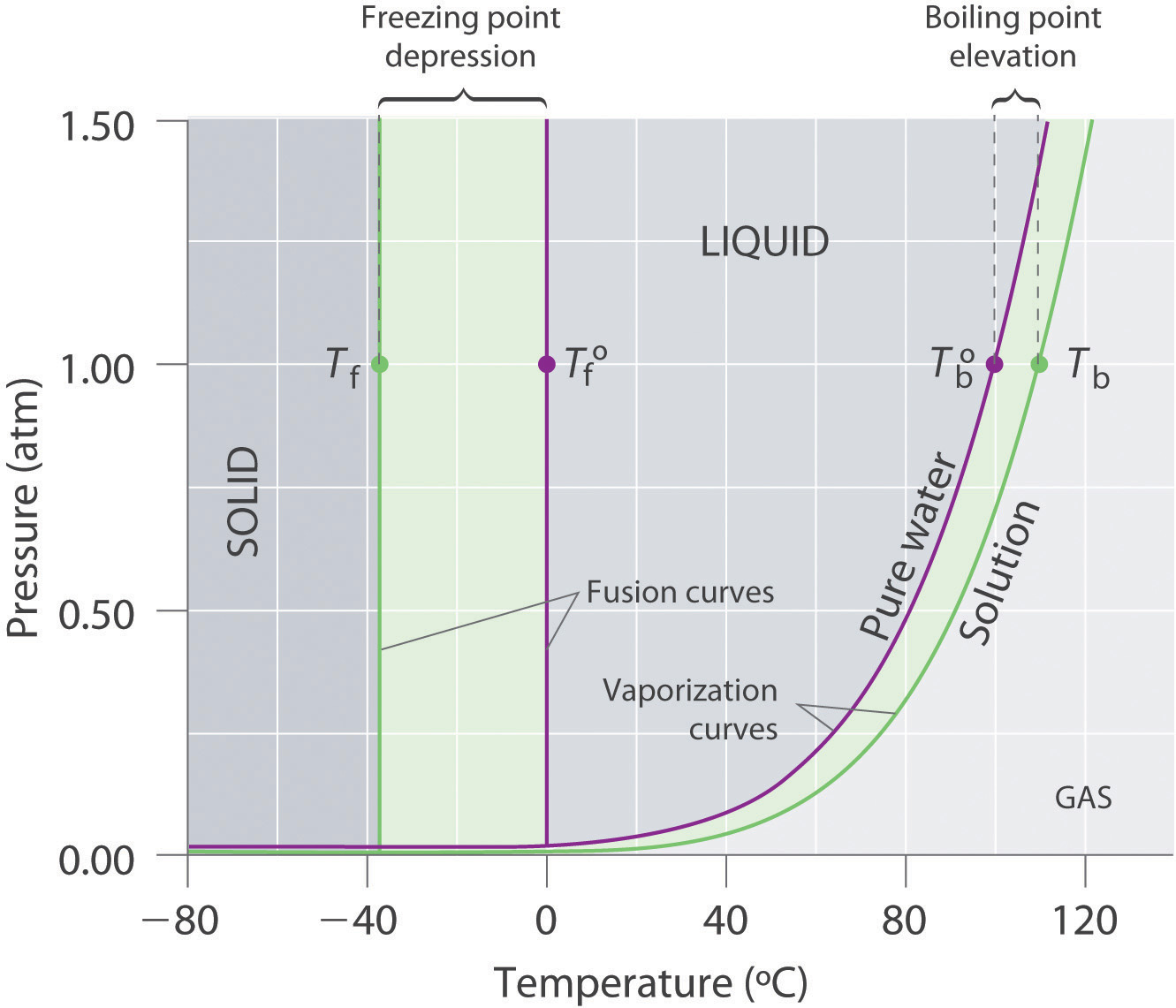
Colligative Properties Of Solutions

Nukiyama S Boiling Curve For Saturated Water At 1 Atm Download Scientific Diagram
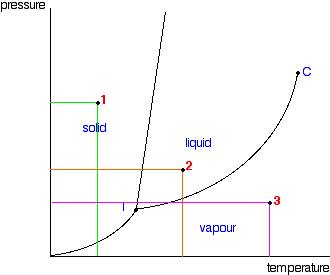
Phase Diagrams Of Pure Substances
At Pressures Greater Than 1 Atm Water Will Boil At のギャラリー
Vapor Pressure Wikipedia

Vapor Pressure And Boiling Youtube
3

Unit 3 States Of Matter Practice Exam Pdf Free Download

Chemistry The Central Science Chapter 13 Section 5
Can Water Always Boil At 100 C Quora
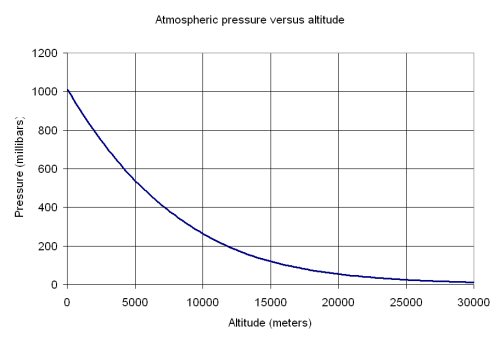
How Do Boiling Points Change At High Altitudes Socratic

0 26 4 The Boiling Point Of Water At 735 Torr Is 99 07 The Mass Of Nacl Added In 100 G Water To Make Its Boiling Point 100 C Is 0 51 K
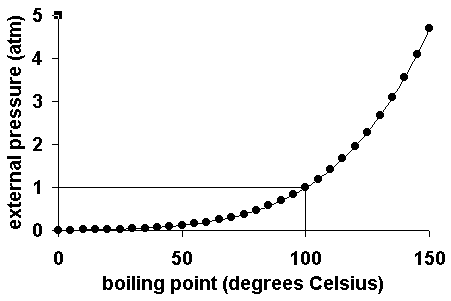
Boiling
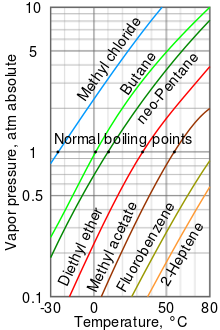
Boiling Point Wikipedia
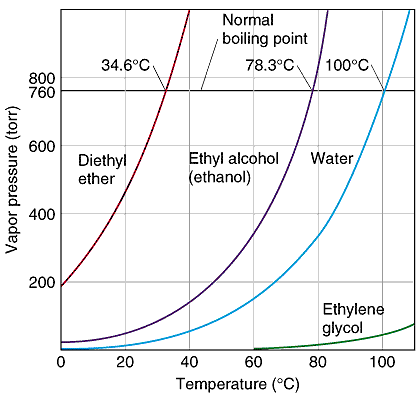
Question d6d Socratic

Boiling Point Of Water At A Place Is Found To Be 110 Oc At This Place

Water Boils At 100 Degree C And 760 Mmhg Water Wi Chegg Com

Distillation
Properties Of Liquids

Solved Question 22 In A Mountain Location Where The Atmos Chegg Com
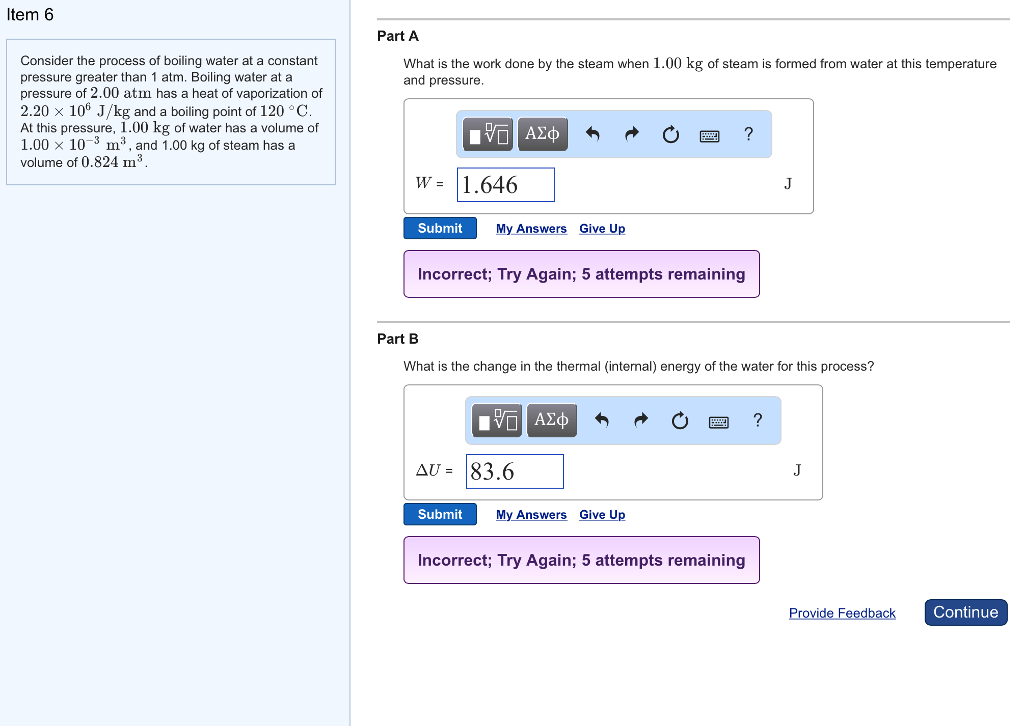
Solved Item 6 Part A Consider The Process Of Boiling Wate Chegg Com

What Temperature Does Water Boil At Boiling Point Elevation Compound Interest

Sublimation Of Iodine Rise And Fall Of A Misconception Chem13 News Magazine University Of Waterloo
Physical Properties Of Liquids A Vapor Pressure
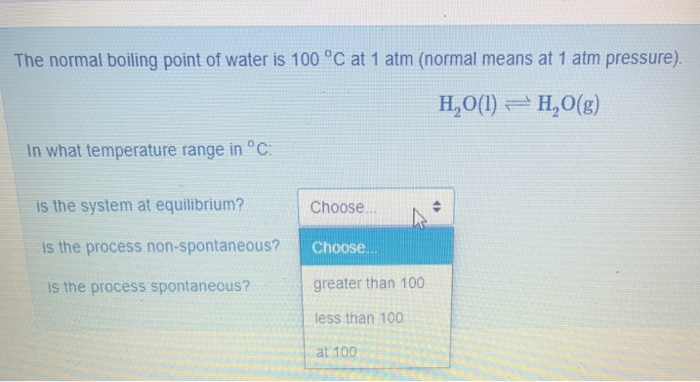
Solved The Normal Boiling Point Of Water Is 100 C At 1 A Chegg Com
Vapor Pressure

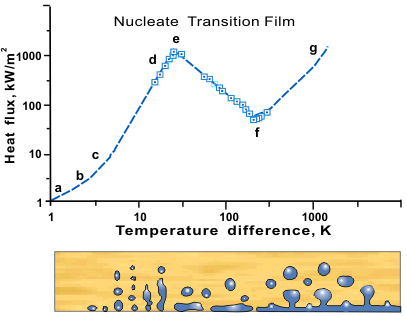
Nucleate Boiling Wikipedia
Vapor Pressure
Boiling Point Wikipedia
10 3 Phase Transitions Chemistry Libretexts
2
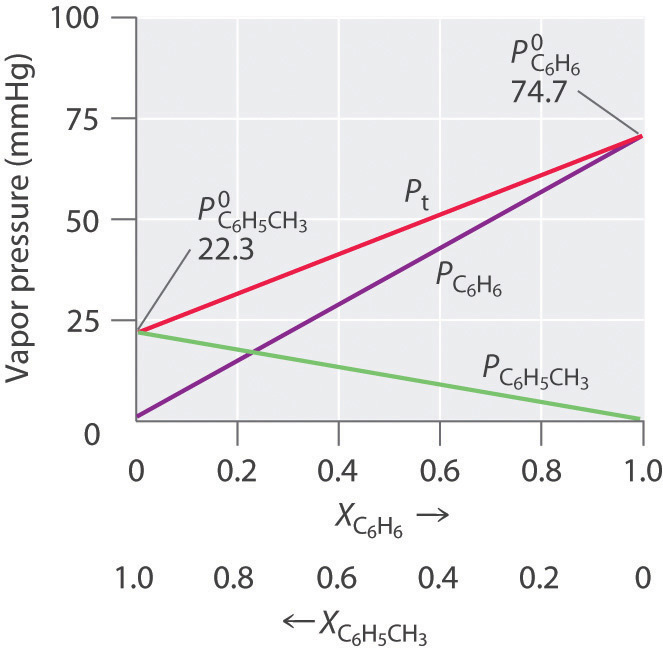
Colligative Properties Of Solutions
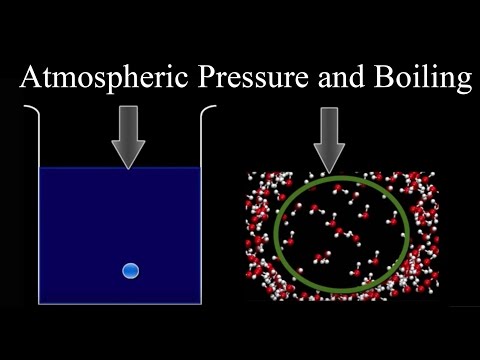
Atmospheric Pressure And Boiling Youtube

Raoult S Law Chemistry Libretexts

Can Water Always Boil At 100 C Quora
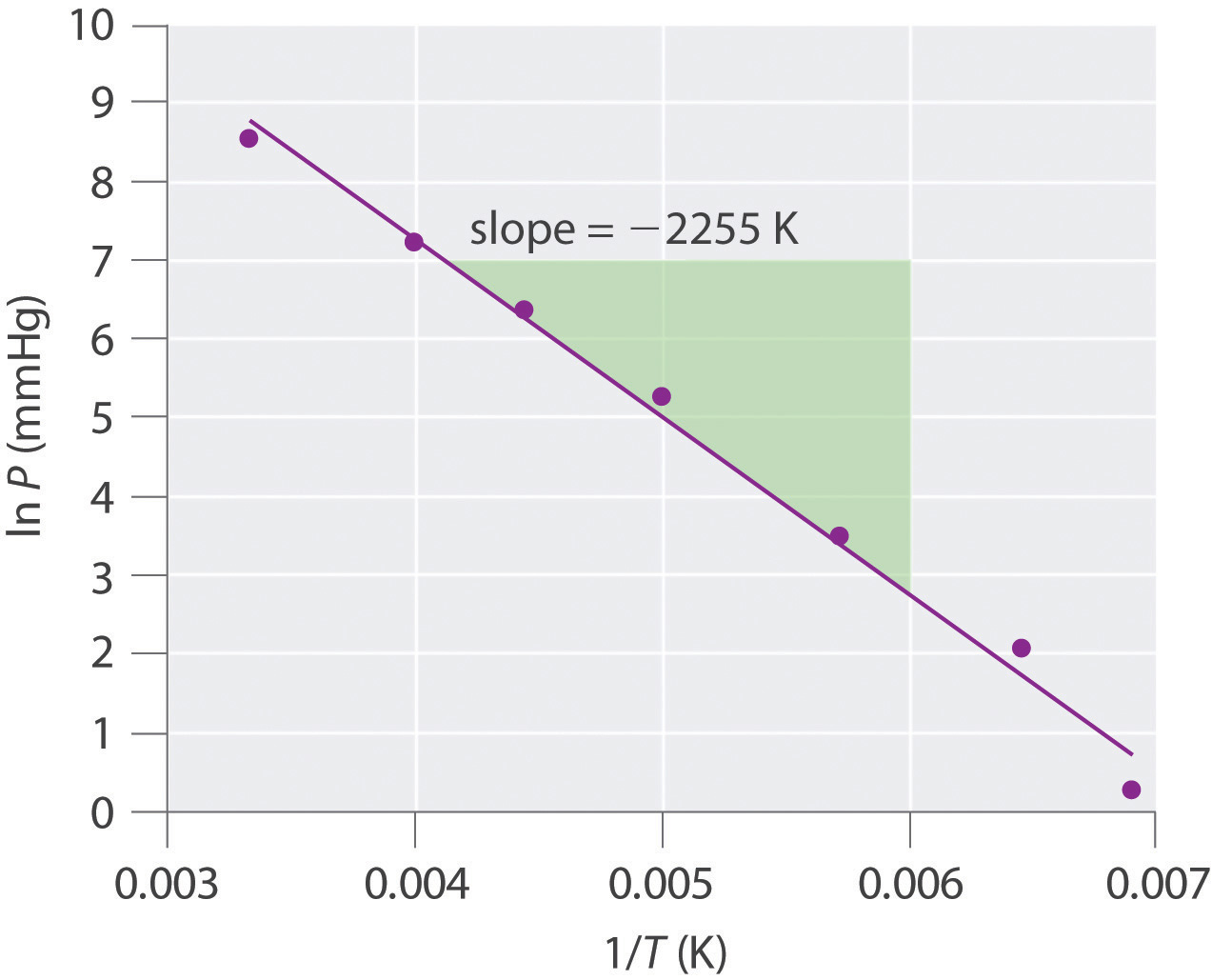
Vapor Pressure
Why Does Any Liquid Boil When Its Vapour Pressure Becomes Equal To Atmospheric Pressure Quora
Physical Properties Of Liquids A Vapor Pressure

8 4 Temperature And Pressure Gay Lussac S Law Ppt Download

Phase Changes Physics
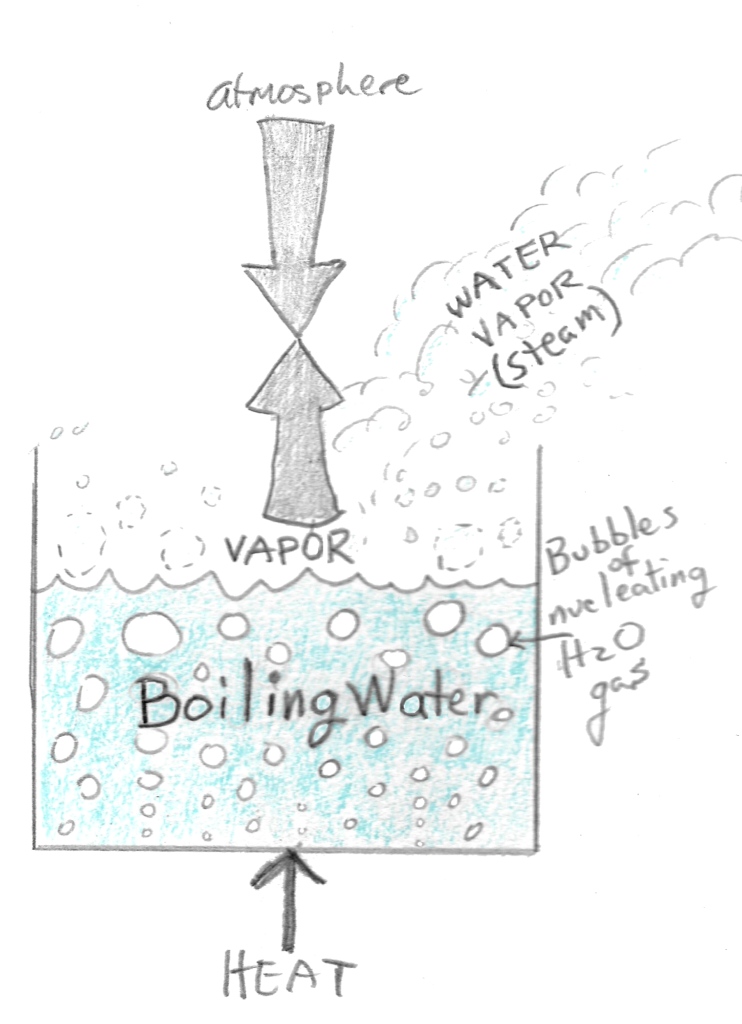
Hard Boiled Eggs Part 1
Hard Boiled Eggs Part 1

Solved Question An Autoclave Is Used To Sterilize Surg Chegg Com

Chem12 C1300 Swbt
What Can Be Said About A Substance With A Triple Point Above 1 Atm Socratic
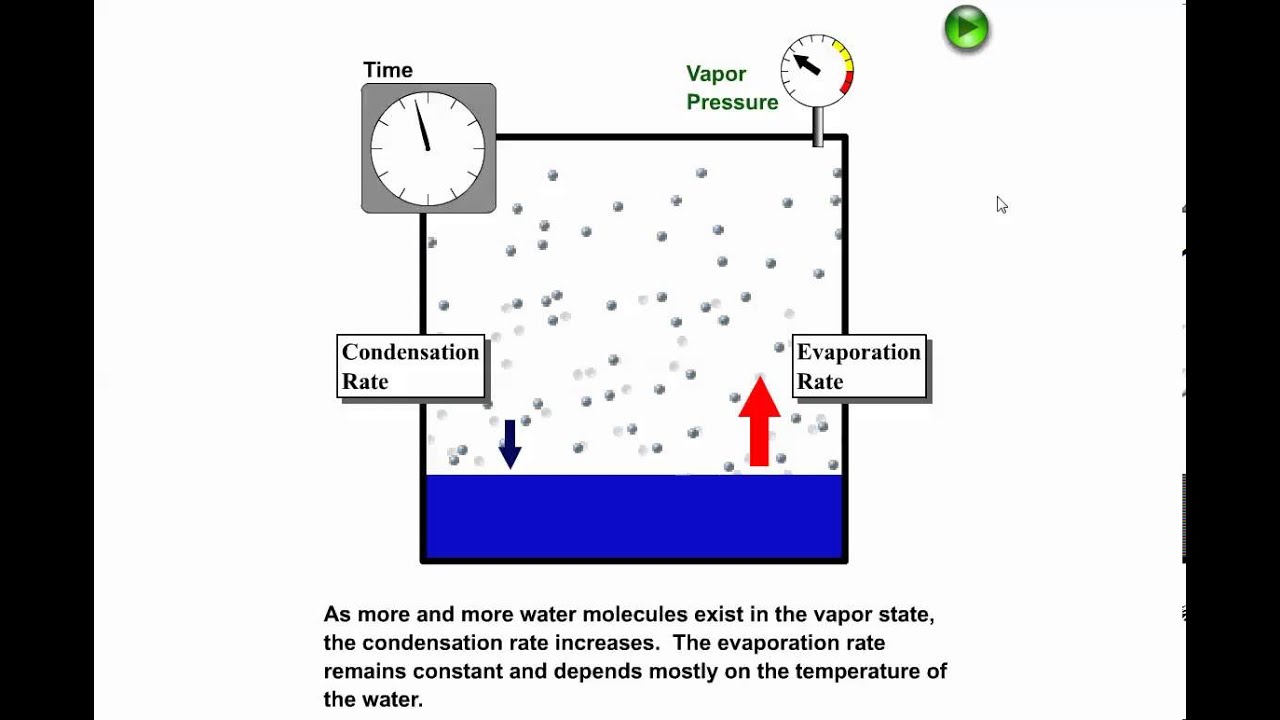
Phase Changes Boundless Chemistry
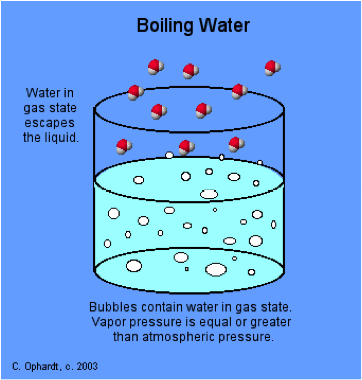
How Does Boiling Point Depend On Pressure And Temperature Socratic
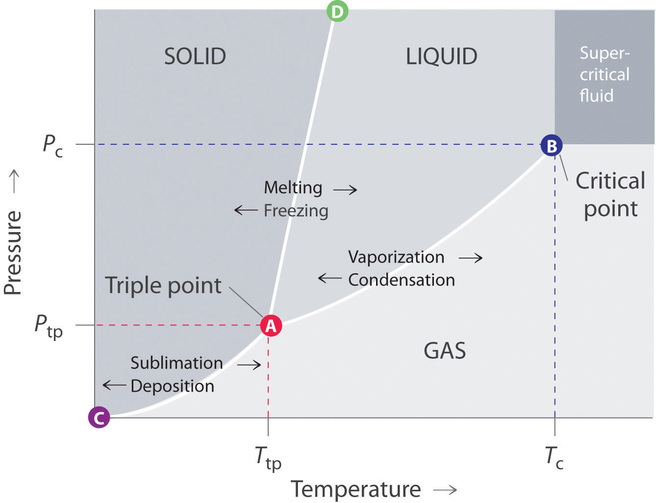
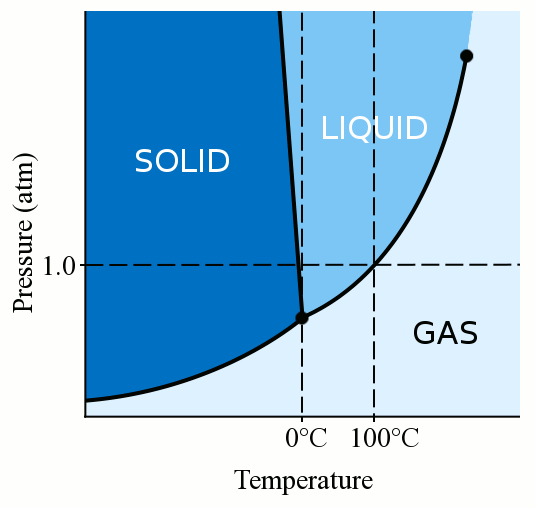
12 4 Phase Diagrams Chemistry Libretexts

The Normal Boiling Point Of Water Is 373 K Vapour Pressure Of Water At Temperature T Is 19 Mm Hg If Enthalpy Of Vaporization Is 40 67 Kj Mole Then Temperature T

Boiling Point Accessscience From Mcgraw Hill Education
.gif)
Q Tbn And9gcrkbmgmtw5b7gyn8jo38gdkh053qoai9ew0yw Usqp Cau
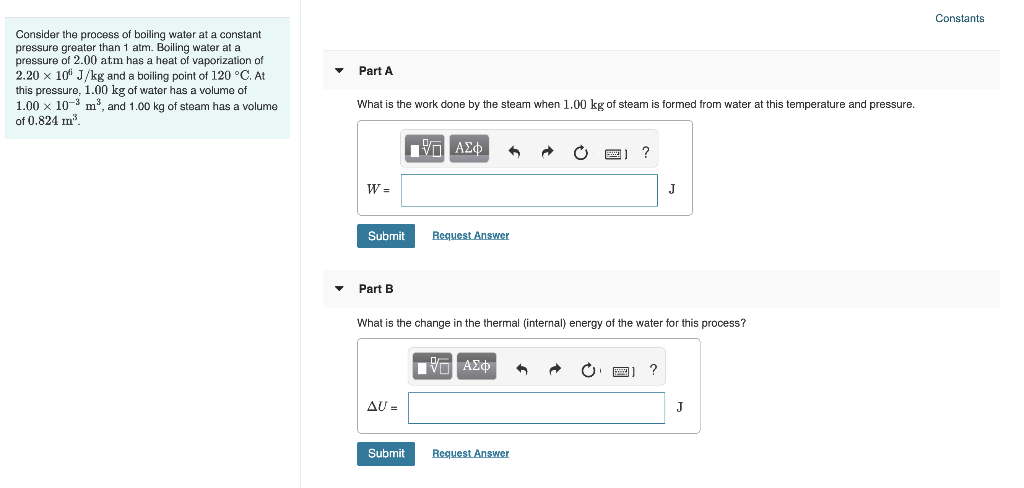
Solved Constants Consider The Process Of Boiling Water At Chegg Com
8 9 Distillation Chemistry Libretexts

7 2 Vaporization Of Liquid Evaporation Basics Learn Tacmina Corporation

Triple Point Wikipedia
Ch150 Chapter 7 Solutions Chemistry
What Is Hotter Boiling Water Or Steam Quora
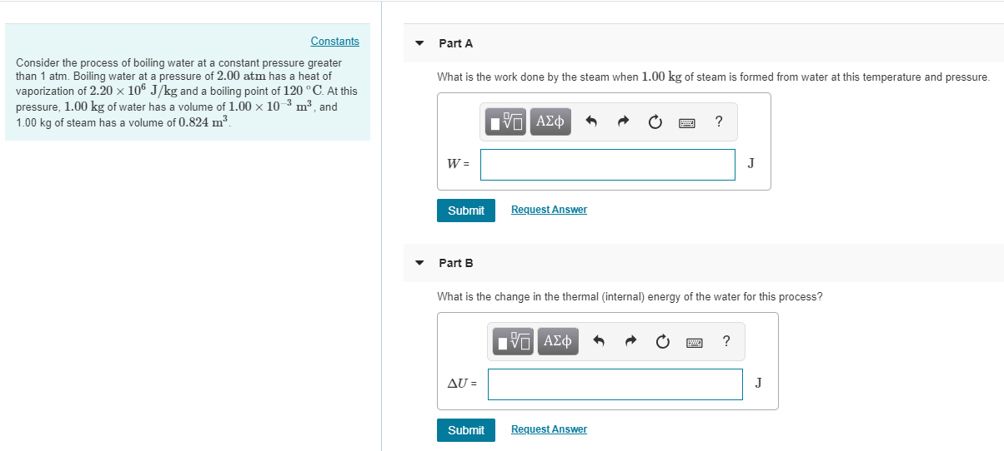
Solved Constants Part A Consider The Process Of Boiling W Chegg Com
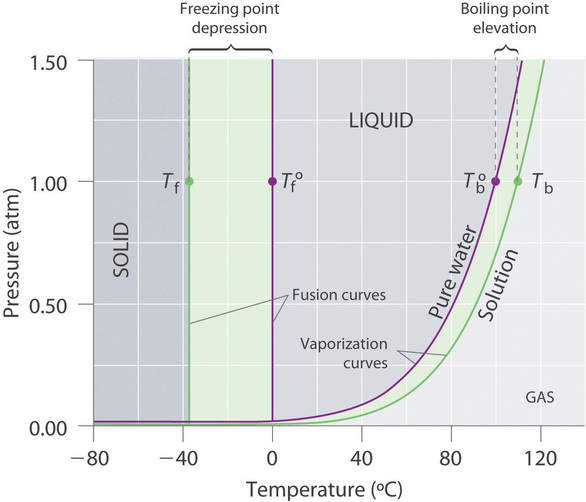
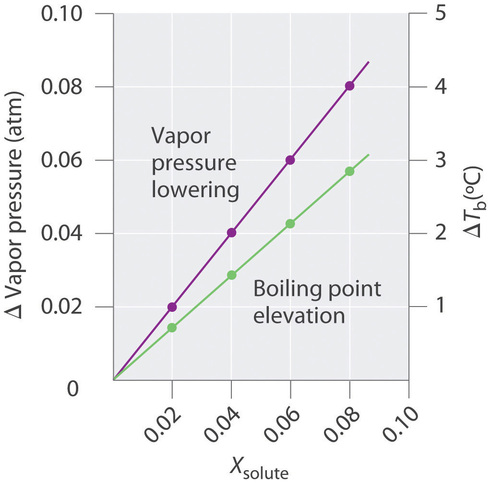
13 8 Freezing Point Depression And Boiling Point Elevation Of Nonelectrolyte Solutions Chemistry Libretexts

Gases And Solutions Test You Ll Remember Quizlet

Phase Diagrams Chemistry For Majors

7 2 Vaporization Of Liquid Evaporation Basics Learn Tacmina Corporation

Why Does Water Boil At Lower Temperature At High Altitudes Quora
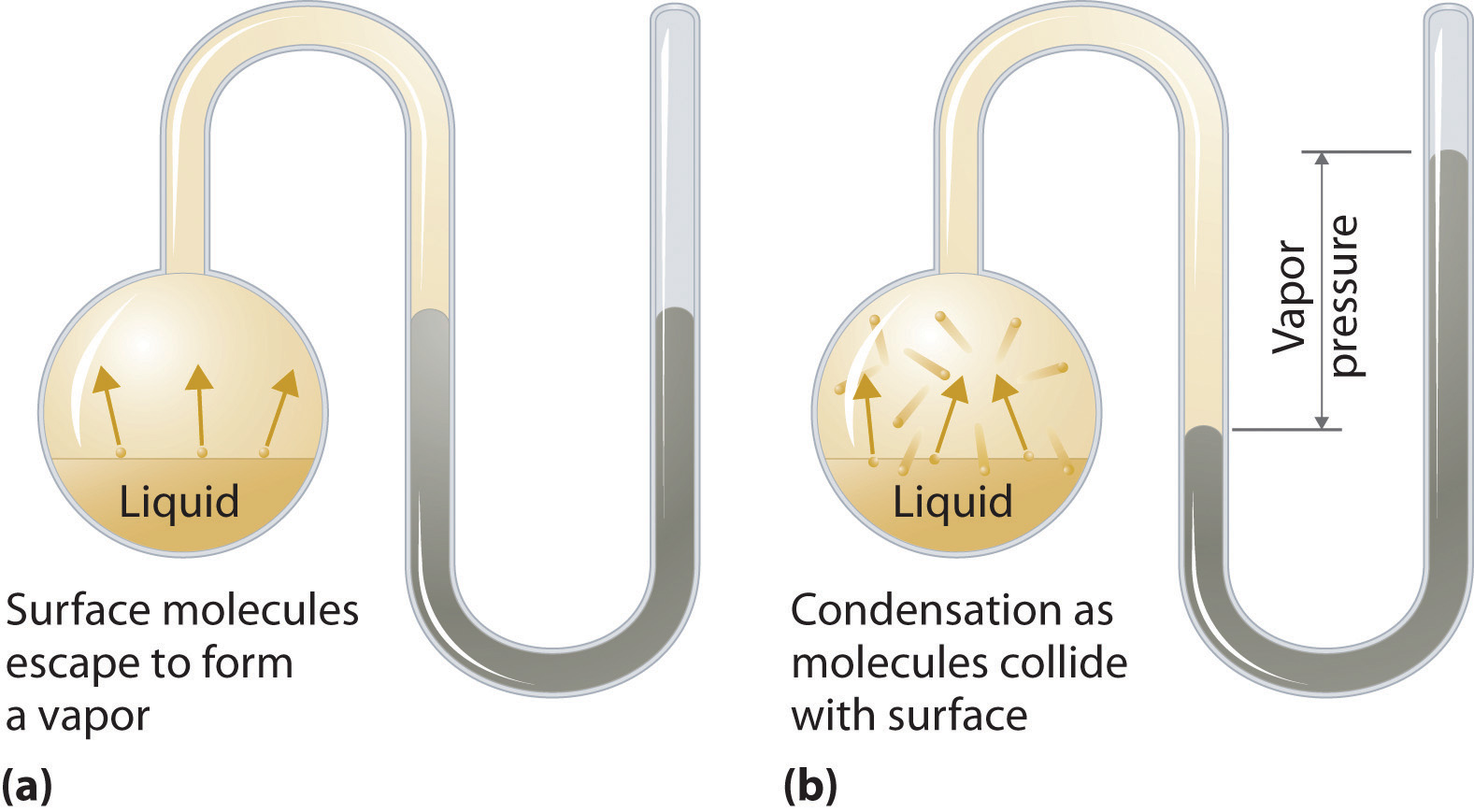
Vapor Pressure
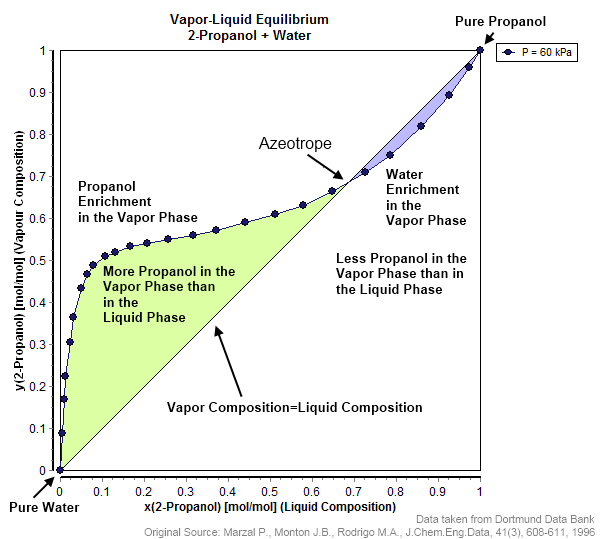
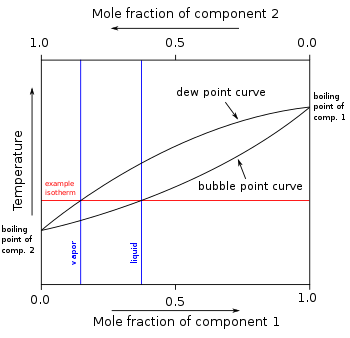
Azeotrope Wikipedia
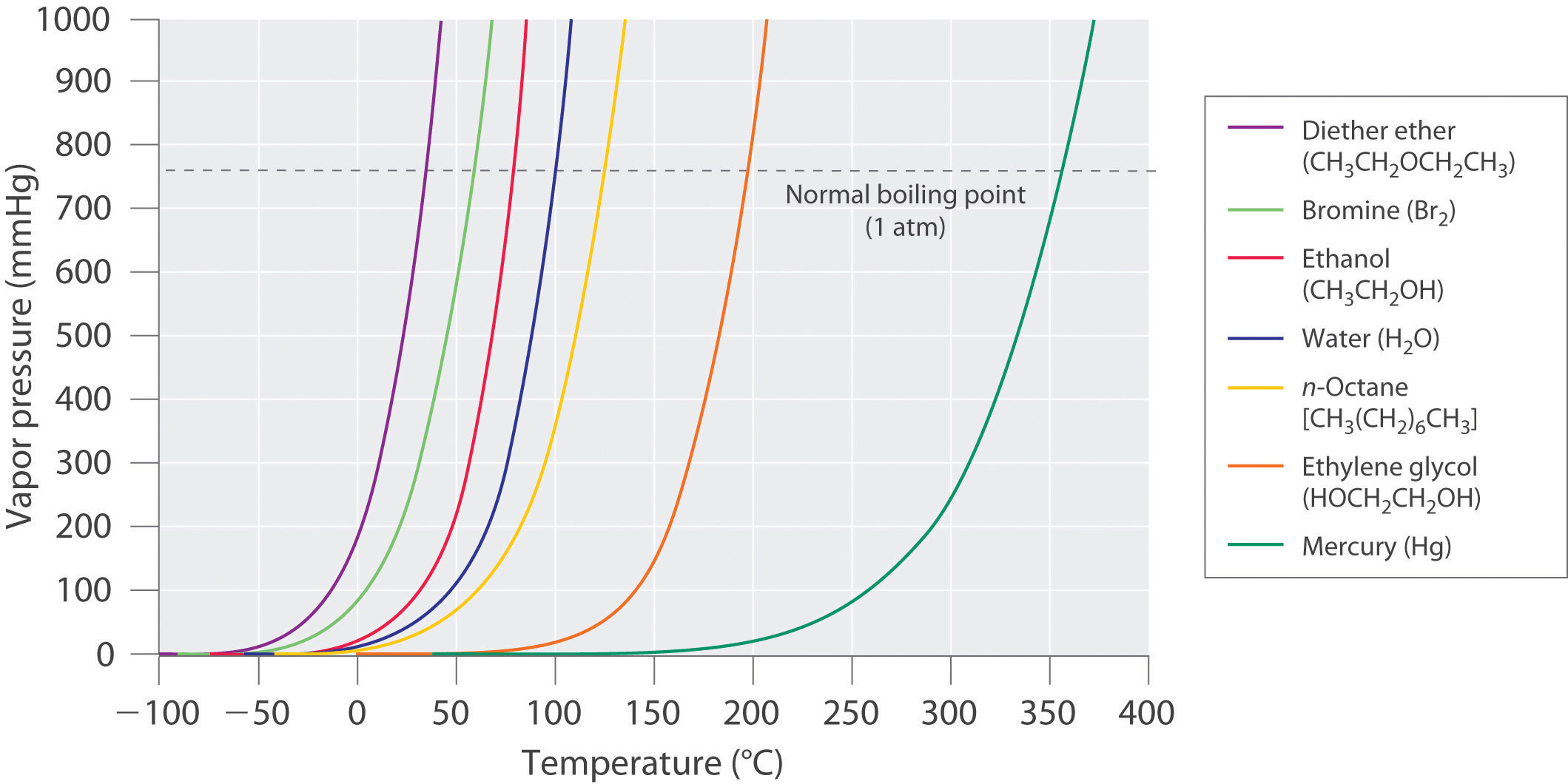
Vapor Pressure
Q Tbn And9gcsfec W9351tx2zvnilhx2ynoarq9istootxx8uvhumk9txjdug Usqp Cau

Family Homecoming Special Event Can Climate Engineering Serve As A Complementary Step To Aggressive Mitigation Dr Michael Maccracken The Climate Institute Ppt Download

What Happens To Boiling Point Of Water In Vacuum Quora

Vapor Pressure And The Ideal Gas Law Worked Example Video Khan Academy
The Earth Introduction

Vapor Pressure Curves Chemistry For Non Majors

Distillation

Solved Name 3 An Increase In The Wemperahure Of A Solu Chegg Com
Can Water Stay Liquid Below Zero Degrees Celsius Science Questions With Surprising Answers
Gases

I Heat Water In An Iron Kettle Is It True That The Iron Of The Kettle Will Never Reach A Temperature Greater Than 100 Degrees Celsius While There Is Water In The
11 5 Phase Equilibrium In Solutions Nonvolatile Solutes Chemistry Libretexts

Best Science Act Set 1 Flashcards Quizlet

11 5 Vapor Pressure Chemistry Libretexts

Chemistry 65
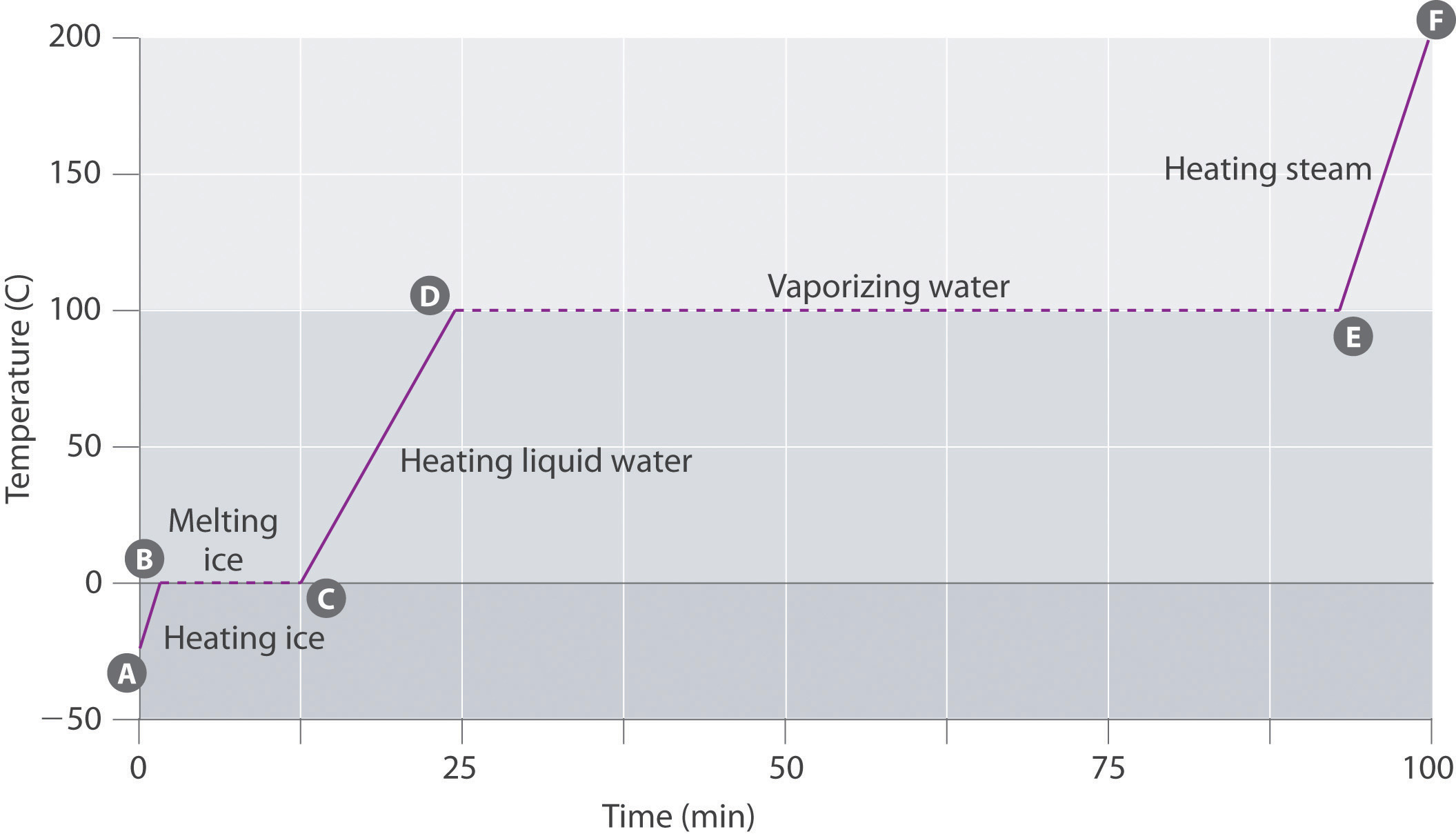
Changes Of State

Phase Changes Physics
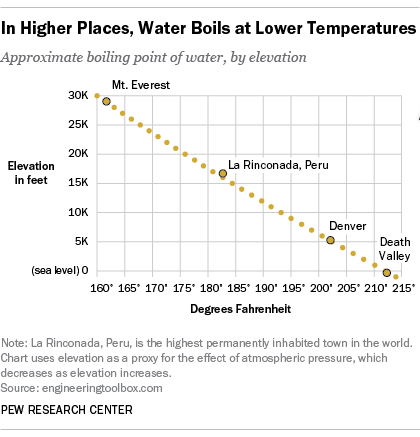
Does Water S Boiling Point Change With Altitude Americans Aren T Sure Pew Research Center
Q Tbn And9gcqhzl5xoury Zprxnqaykg Xfabyj12tmdbi1to7r Sogk7ln Usqp Cau

Solved Which One Of The Following Substances Is Expected Chegg Com
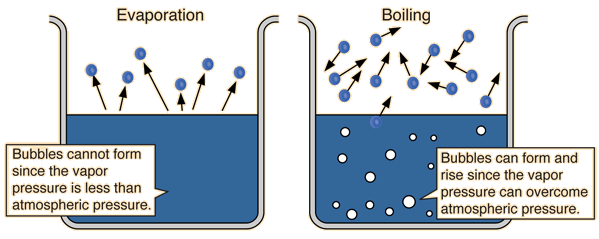
Vapor Pressure

Vapor Pressure And The Ideal Gas Law Worked Example Video Khan Academy
Q Tbn And9gcszse52lexri0yqswezeapghlpdstv1jyd5 Yzbyewoohl7dy3y Usqp Cau

Vapor Pressure Video States Of Matter Khan Academy

If Water Boils At 100 Degrees C At Sea Level How High Must One Be For It To Boil At Zero Degrees C Quora
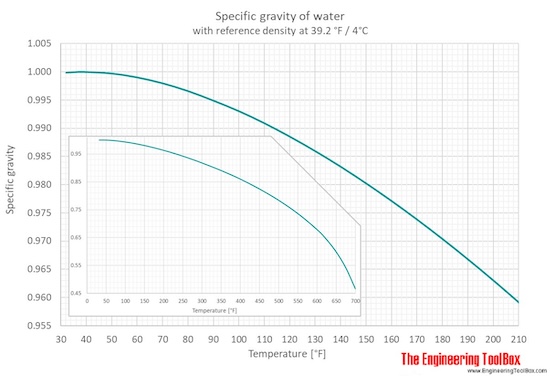
Water Specific Gravity

Phase Changes

Boiling Point Chemistry For Non Majors
Properties Of Liquids

Solved Question An Autoclave Is Used To Sterilize Surg Chegg Com
If Water Boils At 100 Degrees C At Sea Level How High Must One Be For It To Boil At Zero Degrees C Quora
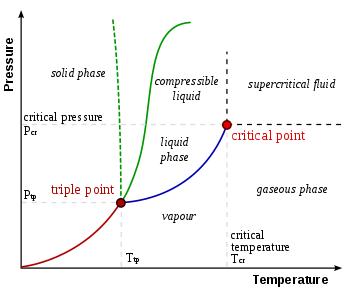
Triple Point Wikipedia
Www Bscsd Org Site Handlers Filedownload Ashx Moduleinstanceid 374 Dataid 1506 Filename Apsolutionspracticeproblemskey061 Pdf
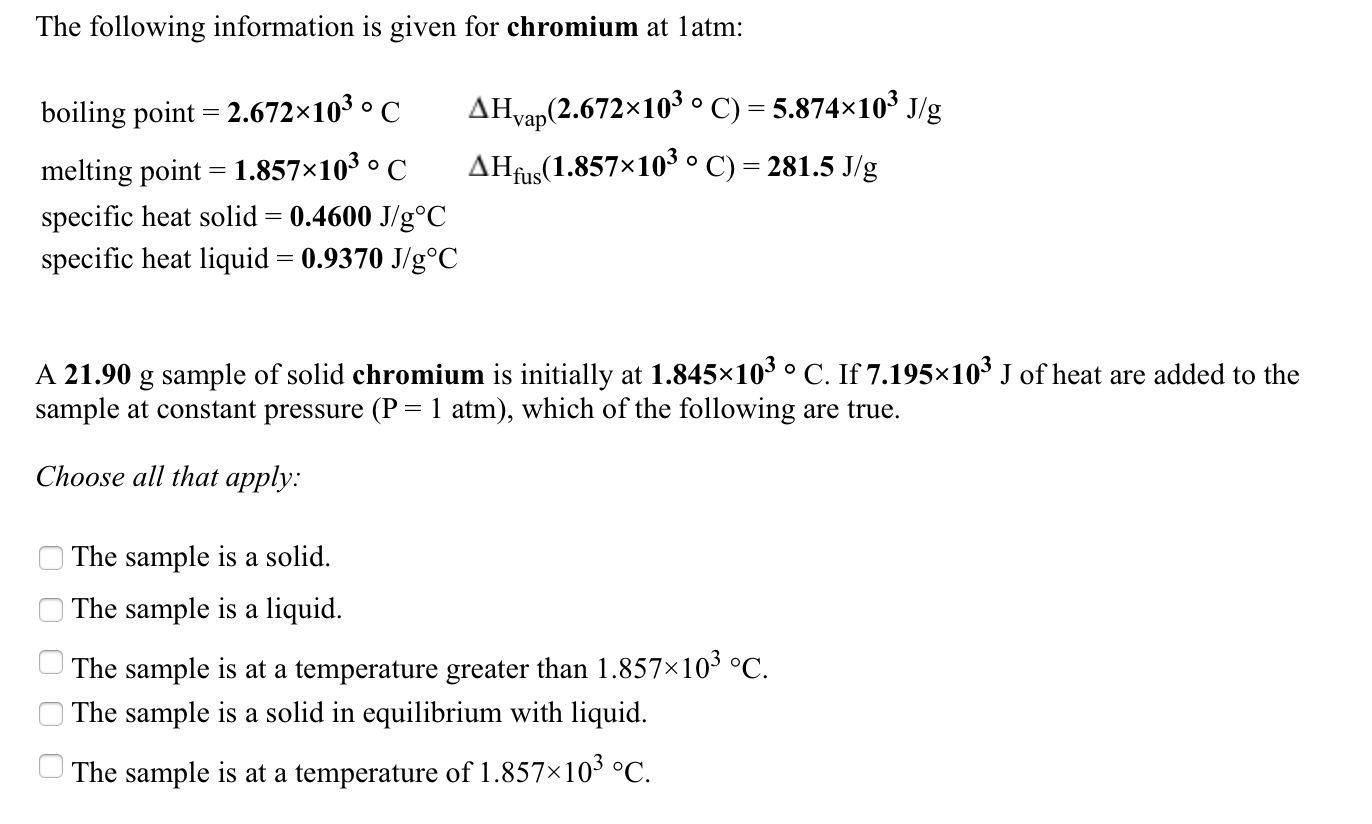
Solved The Following Information Is Given For Water At 1 Chegg Com
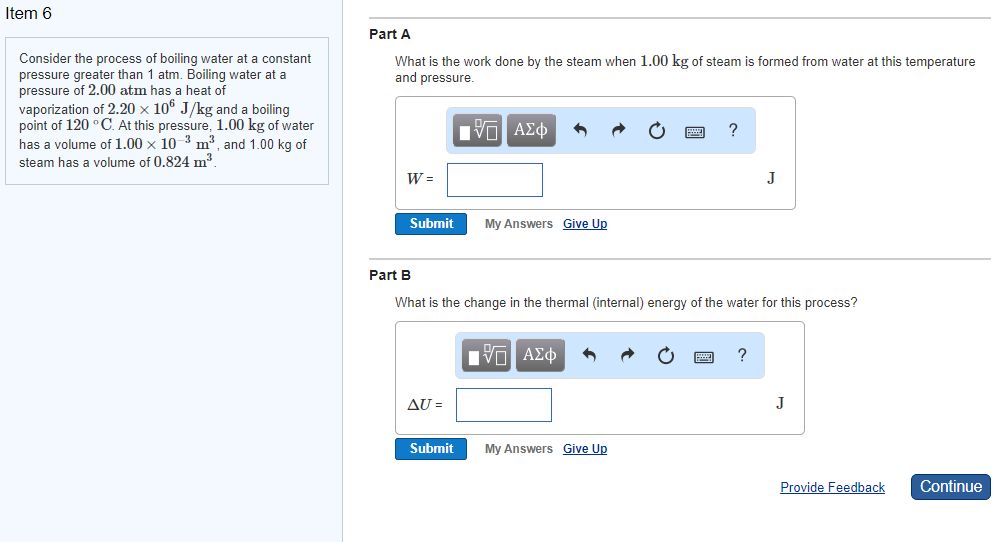
Solved Item 6 Part A Consider The Process Of Boiling Wate Chegg Com
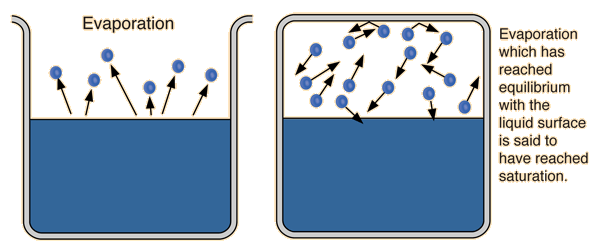
Vapor Pressure

What Temperature Does Water Boil At Boiling Point Elevation Compound Interest

Solved Question An Autoclave Is Used To Sterilize Surg Chegg Com
Boiling



