At Pressures Below Its Triple Point Water Would Sublime
Why is the Triple Point of Water Important.

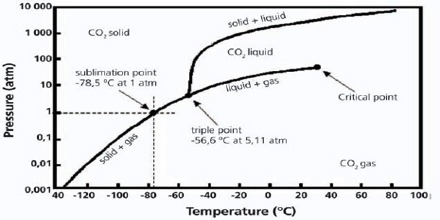
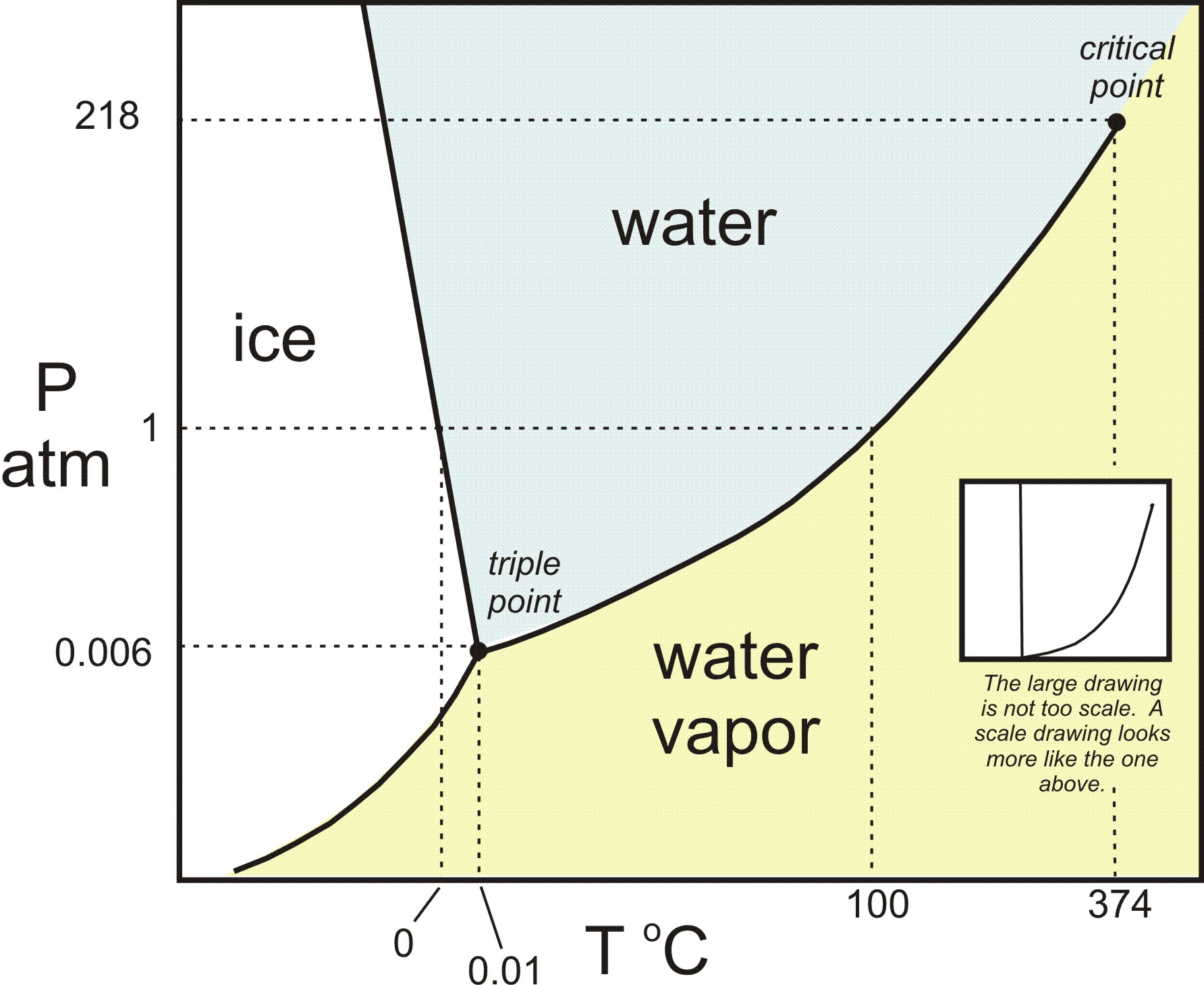
At pressures below its triple point water would sublime. Also, at pressures below the triple point pressure, an increase in temperature will result in a solid being converted to gas without passing through the liquid region. Snow and ice sublime, although more slowly, at temperatures below. Solid carbon dioxide sublimes everywhere along the line below the triple point (e.g., at the temperature of −78.5 °C (194.65 K, −109.30 °F) at atmospheric pressure, whereas its melting into liquid CO 2 can occur only along the line at pressures and temperatures above the triple point (i.e., 5.2 atm, −56.4 °C).
This agreement also sets the size of the kelvin as 1/273.16 of the difference between the triple-point temperature of water and absolute zero. It is also important to note that the triple point of water correlates with the pressure necessary for liquid water to exist. The triple point temperature is – 57 0 C at 5.1 atmospheric pressure where liquid, solid and gaseous CO 2 co-exist.
If the vapor pressure of the ice is higher than the partial pressure of water in the atmosphere the water vapor will enter the atmosphere, and more ice will sublime to try and keep equilibrium. Because ice is less dense than liquid water, ice frozen at pressures below the triple point will sublime directly into water vapor. It is also important to note that the triple point of water correlates with the pressure necessary for liquid water to exist.
Because ice is less dense than liquid water, ice frozen at pressures below the triple point will sublime directly into water vapor. In general, sublimation is a phase change of a substance directly from the solid to the gas phase without passing through the intermediate liquid phase. At pressures below the triple point (as in outer space), solid ice when heated at constant pressure is converted directly into water vapor in a process known as sublimation.
Therefore, the solid sublimes if heated at any pressure below 5.1 atmospheric pressure. At pressures below this value solid and vapour will remain in equilibrium at temperatures indicated by the line along DE. For example, if you were to freeze water into ice and cool it further and then reduce the pressure, you could get ice to sublime.
But at temperatures below that of the triple point, a decrease in pressure will result in a phase transition directly from the solid to the gaseous. At pressures below the triple point (as in outer space), solid ice when heated at constant pressure is converted directly into water vapor in a process known as sublimation. Above the triple point, solid ice when heated at constant pressure first melts to form liquid water, and then evaporates or boils to form vapor at a higher temperature.
Water as a liquid does not sublime (go from solid to gas), but water ice does.

Uranium Hexafluoride Source Appendix A Of The Peis Doe Eis 0269 Physical Properties
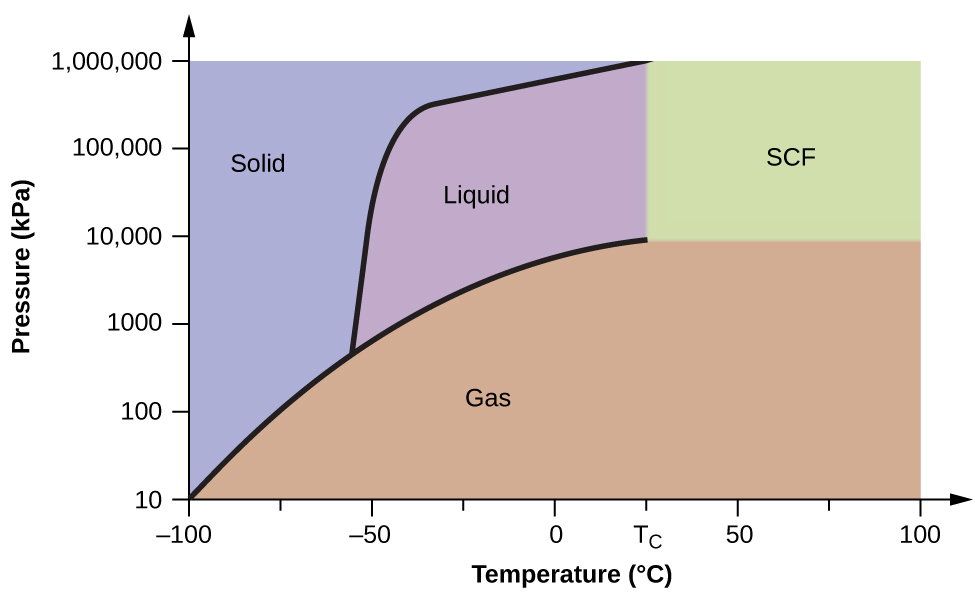
Phase Diagrams Chemistry Atoms First 2e
Sublimation In Triple Point Qs Study
At Pressures Below Its Triple Point Water Would Sublime のギャラリー
3
What Can Be Said About A Substance With A Triple Point Above 1 Atm Socratic

Best Kinetic Molecular Theory Flashcards Quizlet
Http Msadrangi Weebly Com Uploads 8 8 5 0 5032 Phase Diagram Worksheets Answers Pdf

Melting Point Wikipedia

Solid To Gas Phase Transition Introduction To Chemistry

Chemistry The Central Science Chapter 11 Section 6

Phase Diagrams Chemistry Atoms First Openstax Cnx

Lab Dry Ice Wp Edline Pages 1 4 Flip Pdf Download Fliphtml5

Does Everything Exist As A Gas At P 0 Chemistry Stack Exchange
Q Tbn And9gcta7hoxw4a2uhbe6nrmzs2pk2jlhgjcbi3vpnqh90dasq2ycxgk Usqp Cau
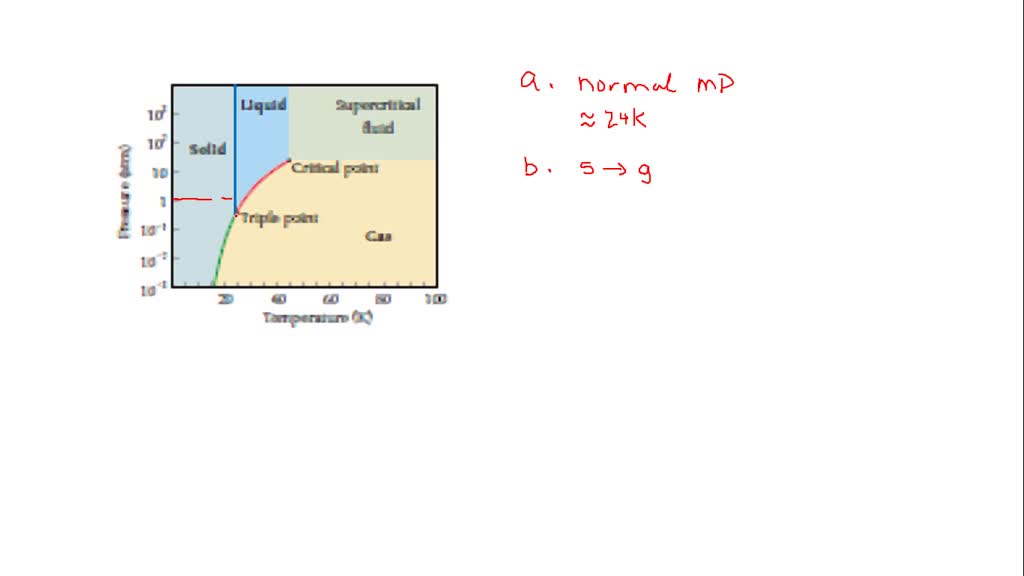
Solved The Phase Diagram For Neon Is Use The Phas

Freeze Drying Lyophilization Information Basic Principles

Answers To Selected Problems Chapter 11

Phase Diagrams

Sublimation Of Iodine Rise And Fall Of A Misconception Chem13 News Magazine University Of Waterloo
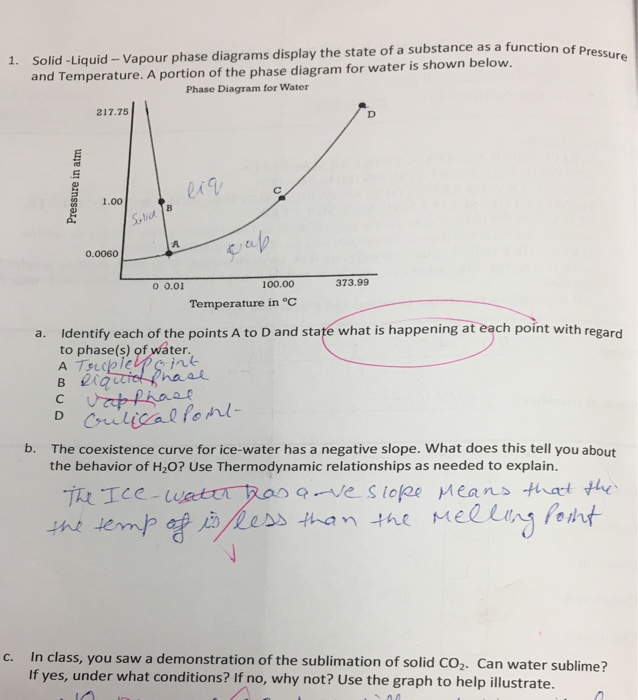
Solved Solid Liquid Vapour Phase Diagrams Display The St Chegg Com
Ucsb Science Line
Vapor Pressure Curves
Sublimation In Triple Point Qs Study
Phase Diagram Of Carbon Dioxide C11 1 01 05
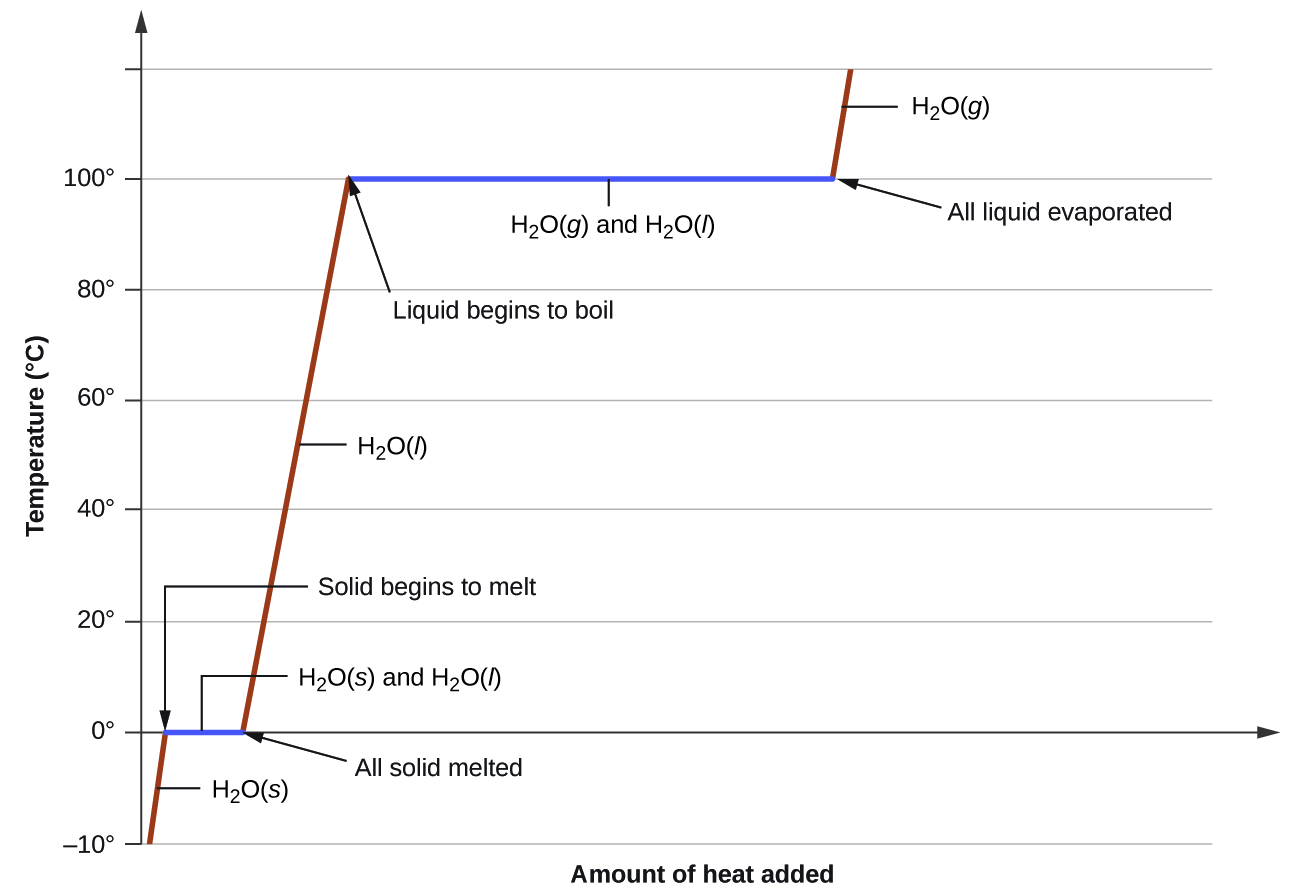
8 1 Heating Curves And Phase Changes Chemistry Libretexts
Q Tbn And9gcspkygotpwl9wyarcwdcxw280zptloxz Pjb5pflzx7t9fcewnm Usqp Cau
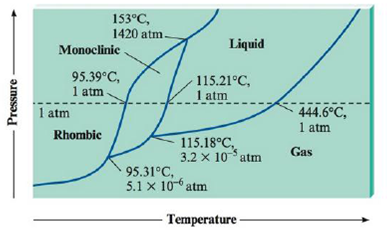
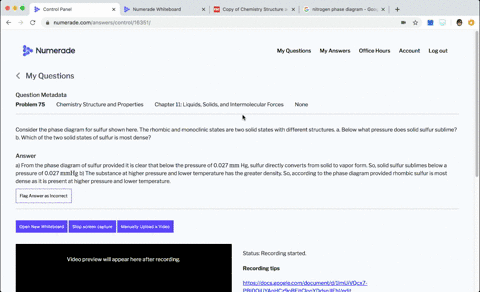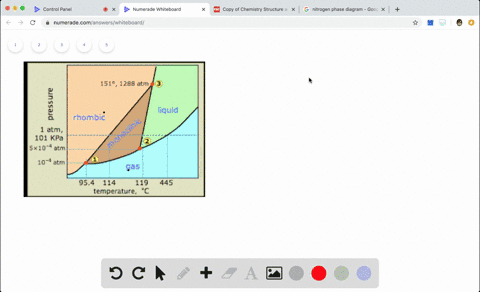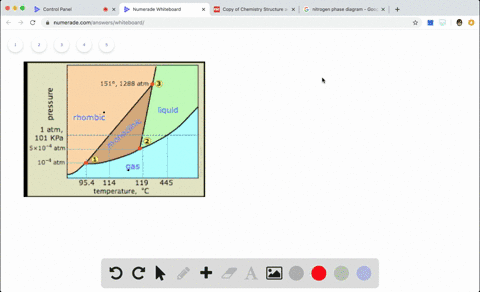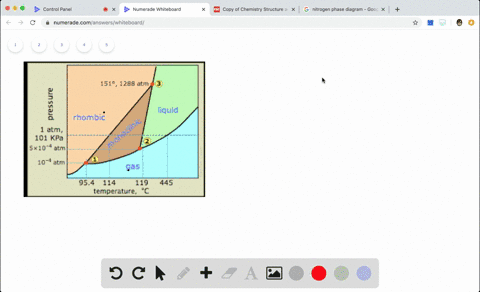
Sulfur Exhibits Two Solid Phases Rhombic And Monoclinic Use The Accompanying Phase Diagram For Sulfur To Answer The Following Questions The Phase Diagram Is Not To Scale A How Many Triple Points

Sublimation Phase Transition Wikipedia

Phase Changes Boundless Chemistry

Phase Diagrams Ck 12 Foundation

12 4 Phase Diagrams Chemistry Libretexts
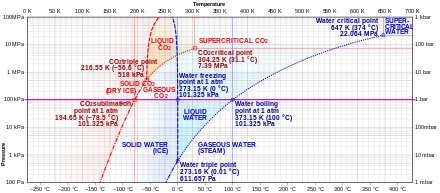
Sublimation Phase Transition Wikipedia

Physicshelponline Phase Change

Phase Changes Vapor Pressure General Chemistry Lecture 1140 Dr Sundin Uw Platteville
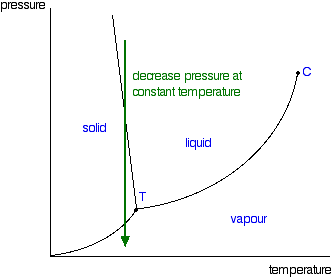
Phase Diagrams Of Pure Substances
Simple Small Scale Dry Ice Explosions Chemical Education Xchange
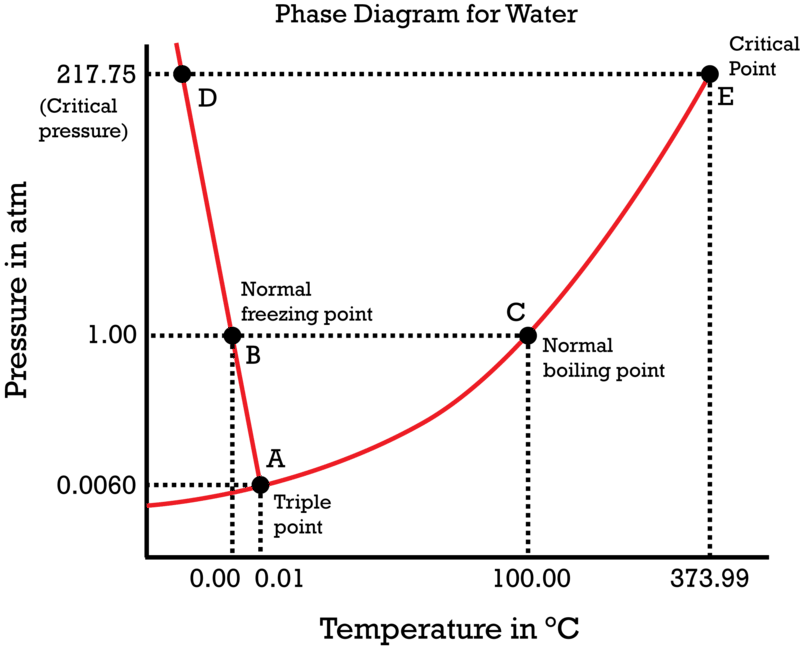
Phase Diagrams Ck 12 Foundation

Pure Substances Vtu E Learning Centre Pages 1 39 Flip Pdf Download Fliphtml5
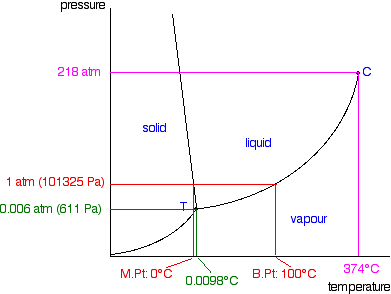
Phase Diagrams Of Pure Substances
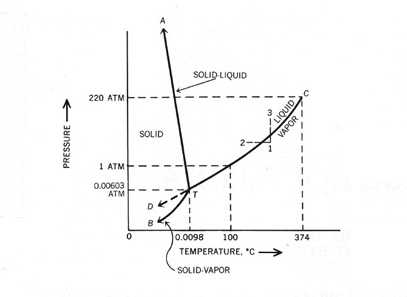
Triple Point

Sublimation An Overview Sciencedirect Topics
Is Carbon Dioxide The Only Compound To Sublime Solid To Gas Phase Transition Skipping Liquid Phase Quora

Liquid Behaviour Of Pure Liquids Britannica
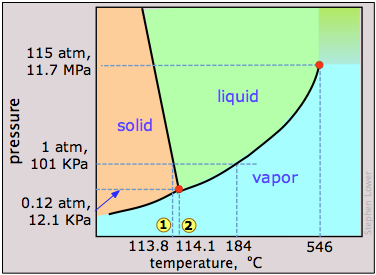
7 5 Changes Of State Chemistry Libretexts

Pdf On The Possibility Of Liquid Water On Present Day Mars

Phase Diagrams Chemistry Atoms First Openstax Cnx

What Compounds Or Elements Only Have One Phase Or Two Phases Physics Stack Exchange
Understanding Phase Changes And Heating Curves
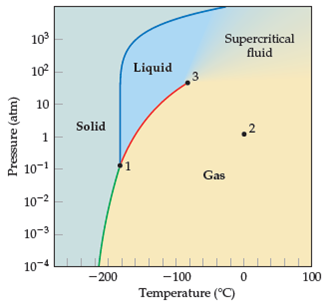
Solved Interpreting A Phase Diagramuse The Phase Diagram For M Chegg Com
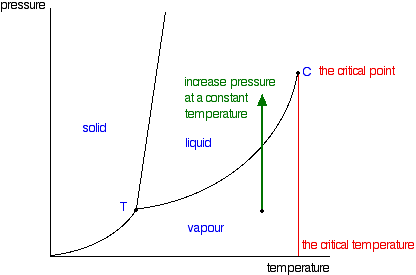
Phase Diagrams Of Pure Substances
Http Arulscience Weebly Com Uploads 8 7 8 4 Extra Practice Phase Diagrams 3 Answer Key Pdf
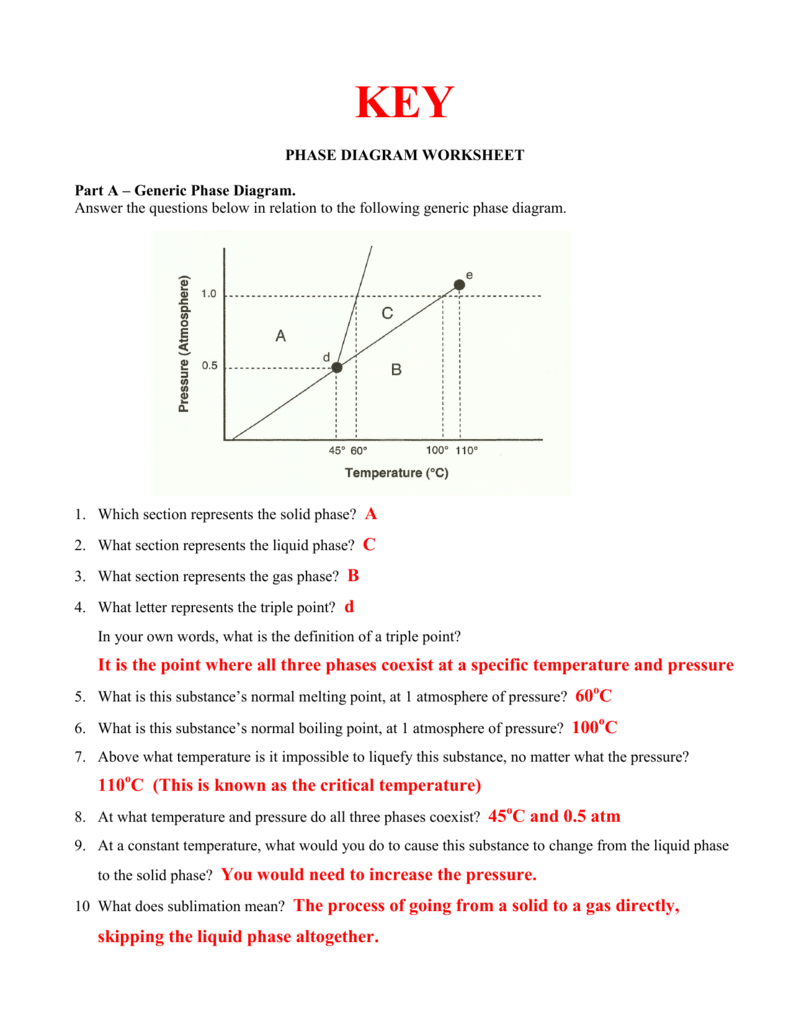
Phase Diagram Worksheet
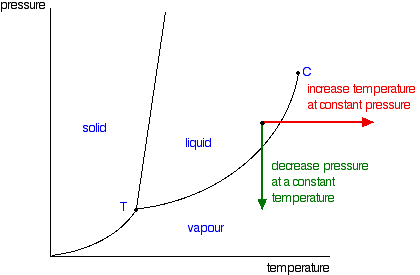
Phase Diagrams Of Pure Substances

Phase Diagrams Ck 12 Foundation

Use The Phase Diagram For Neon To Answer T Clutch Prep

Phase Changes Boundless Chemistry
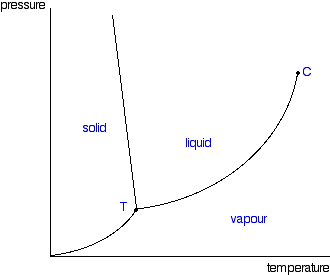
Phase Diagrams Of Pure Substances
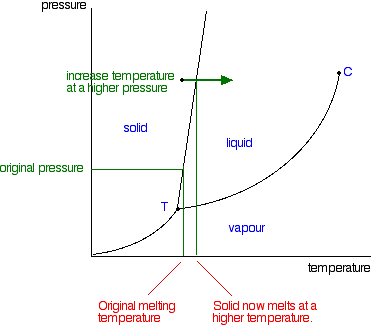
Phase Diagrams Of Pure Substances

Phase Diagrams Chemistry Atoms First Openstax Cnx
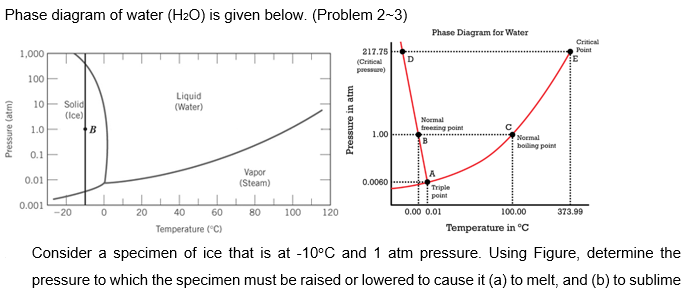
Phase Diagram Of Water H2o Is Given Below Prob Chegg Com
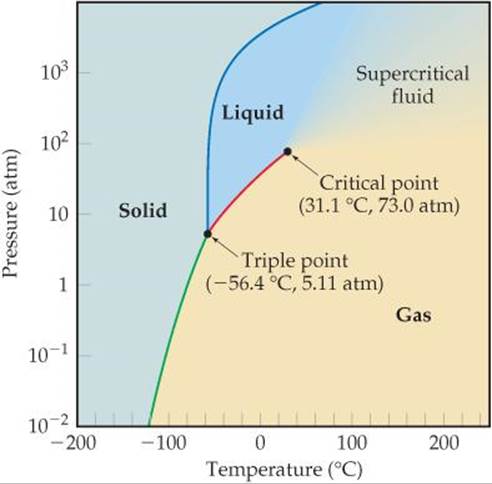
Phase Diagrams Liquids And Intermolecular Forces Chemistry The Central Science

Q A Sublimation Department Of Physics University Of Illinois At Urbana Champaign
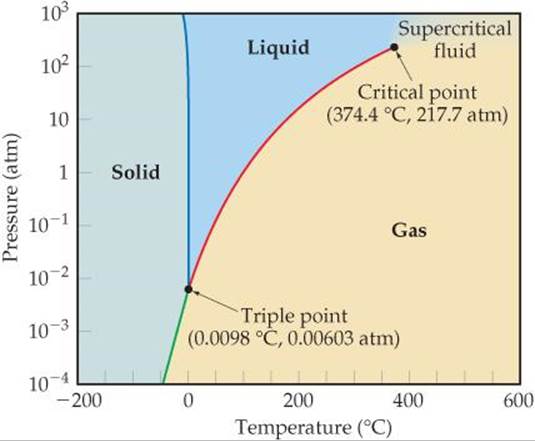
Phase Diagrams Liquids And Intermolecular Forces Chemistry The Central Science
Understanding Phase Changes And Heating Curves

Q Tbn And9gcscfun Elelokjen98tdadrpip Roodjn74sg Usqp Cau
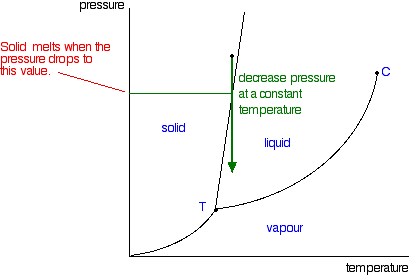
Phase Diagrams Of Pure Substances
Why Don T All Substances Undergo Sublimation Quora

Changes Of State Ck 12 Foundation
Www Cardozohigh Com Ourpages Auto 15 10 Hw 8 triple point Pdf
Www Lamar Edu Arts Sciences Files Documents Chemistry Biochemistry Dorris 1412review2 Pdf
Is Carbon Dioxide The Only Compound To Sublime Solid To Gas Phase Transition Skipping Liquid Phase Quora

Phase Changes

Use The Phase Diagram For Neon To Answer T Clutch Prep
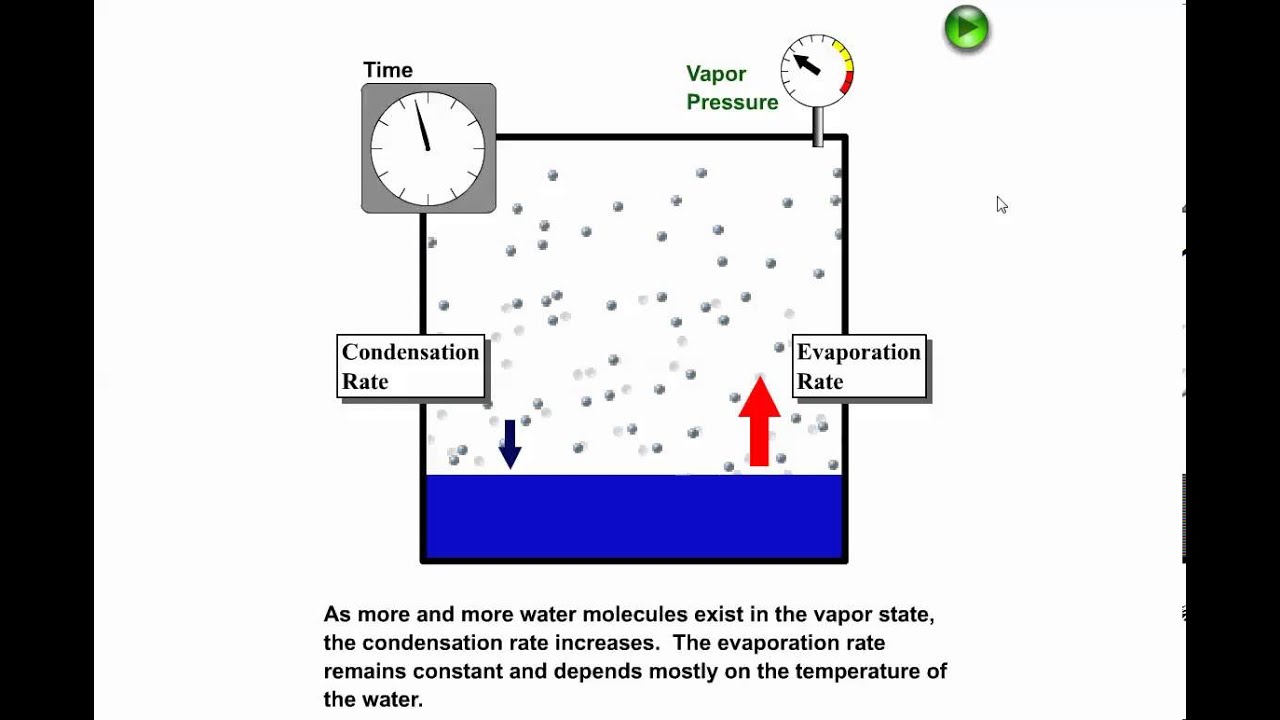
Phase Changes Boundless Chemistry
Q Tbn And9gcrnrrio2zh8hqbe7vsixx3jxsu X Rbrlemsa Usqp Cau
Www Spscientific Com Workarea Downloadasset Aspx Id 1318

Phase Diagrams Video States Of Matter Khan Academy

Sketch A P T Phase Diagram For A Substance Which Sublimates Under Normal Conditions Room Temperature And 1 Atm What S The Essential Requirement For This To Happen Study Com

Phase Diagrams Of Pure Substances A Level Chemistry Revision Xtremepapers Advancing Knowledge Is In Our Dna

Liquid Water And Life On Mars
Haddonfield Instructure Com Files Download Download Frd 1

10 4 Phase Diagrams General College Chemistry I

Why Do Some Substances Undergo Sublimation Why Don T They Have A Liquid Phase Quora

The Triple Point Of Water Is 0 0098 C At 0 Atm 4 58 Clutch Prep
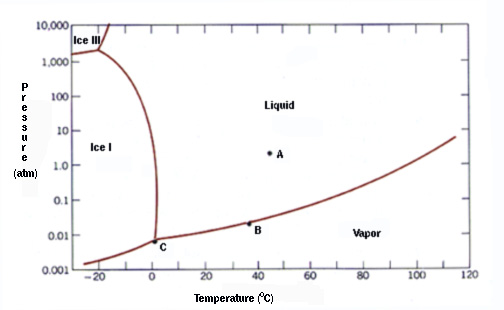
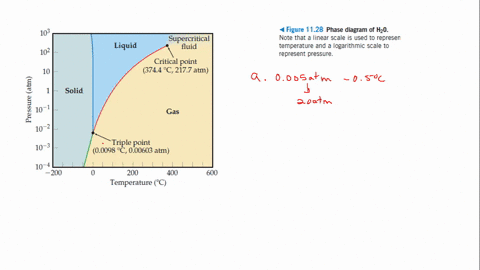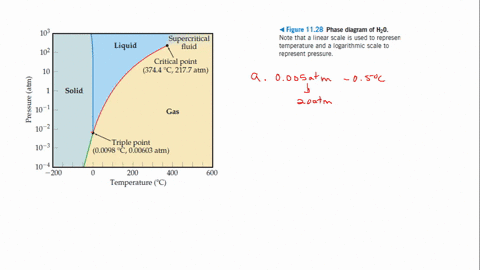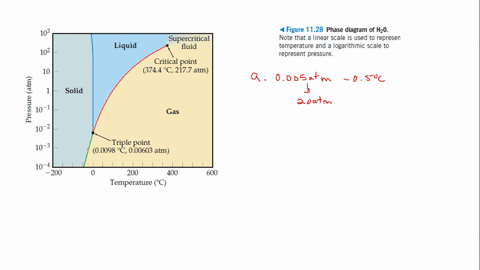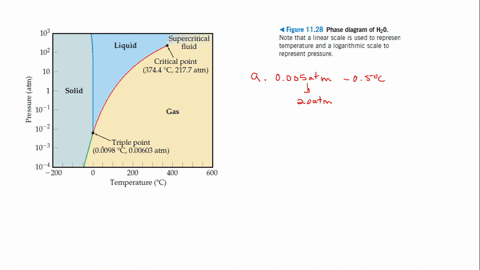
Example Problems Involving The Triple Point Of Water

Chemistry The Central Science Chapter 11 Section 6

Triple Point Of Water High Accuracy Temperature Probes

Phase Diagrams Of Pure Substances A Level Chemistry Revision Xtremepapers Advancing Knowledge Is In Our Dna

Chapter 11 Section 6
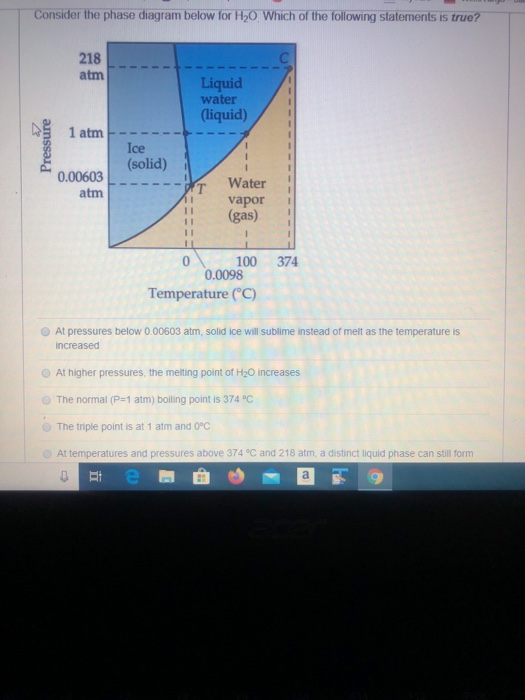
Solved Consider The Phase Diagram Below For H2o Which Of Chegg Com

The Phase Diagram For A Pure Substance Is Shown Above Use This Diagram And Your Knowledge About Changes Of Phase To Answer The Following Questions If The Triple Point Pressure Of A Substance

On The Basis Of The Following Figure What Is The Form Of A Substance In The State B
Q Tbn And9gct7zndvuqa Wmz8rlvy7aztpieg0oah9vn Eg Usqp Cau

Why Do Some Substances Undergo Sublimation Why Don T They Have A Liquid Phase Quora

Phase Diagrams Chemistry Libretexts

Triple Point Of Water High Accuracy Temperature Probes
Chem11 Chapter 11 Quiz 1
Scilearn Sydney Edu Au Firstyear Exams Chem1102 Topic19 Answers Pdf
Http Www Kengoldsby Com Wp Content Uploads 02 Gen Chem Ii Solutions To Practice Problems 1 34 Pdf

The Mars Ice Faq How Do You Know It S Water Wired
Q Tbn And9gcrsofoj Qajuddwvckrtxwwntonjy5fxvwmg1uay1j 7udqgn Usqp Cau
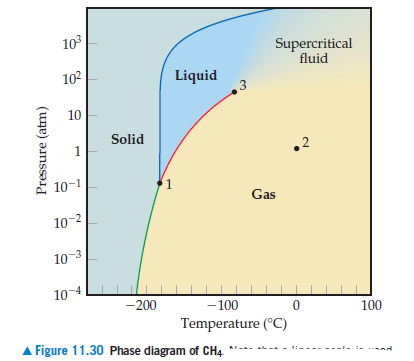
Answered 10 Supercritical Fluid 102 Liquid 10 Bartleby

Phase Changes
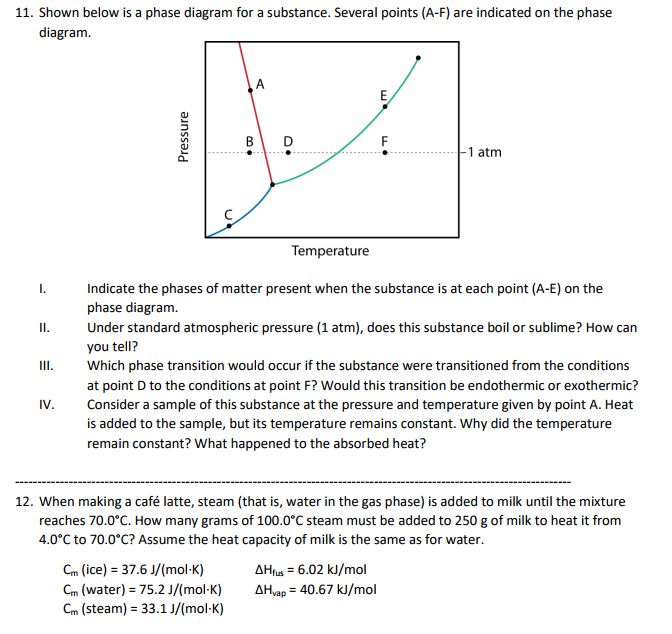
Solved 11 Shown Below Is A Phase Diagram For A Substance Chegg Com



